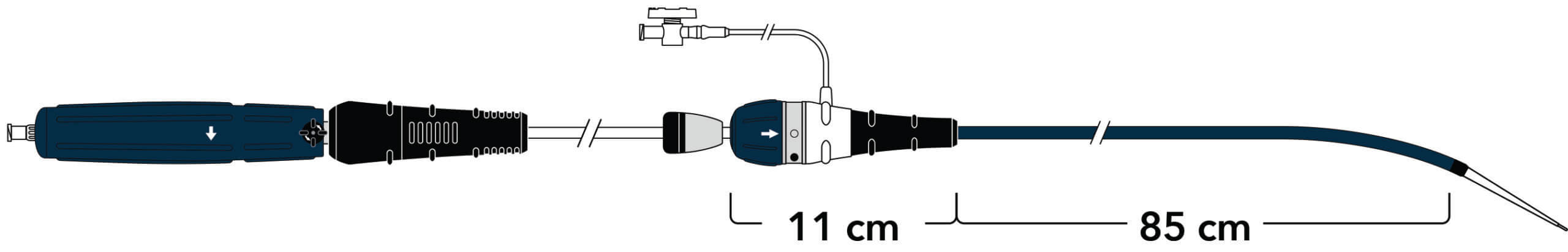
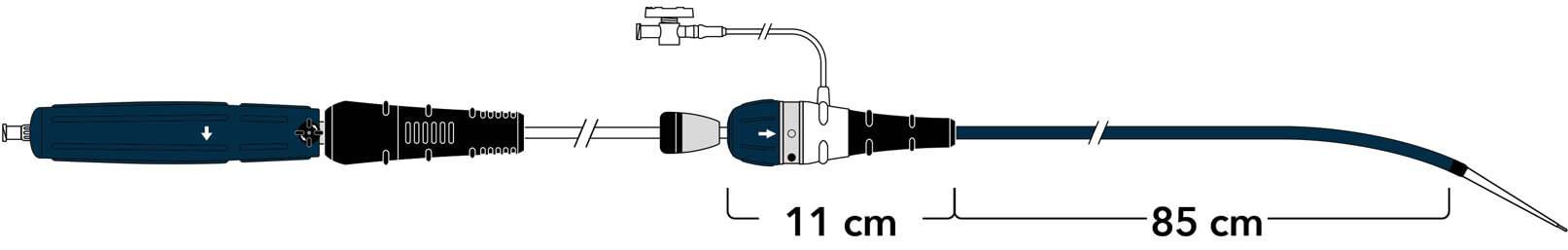
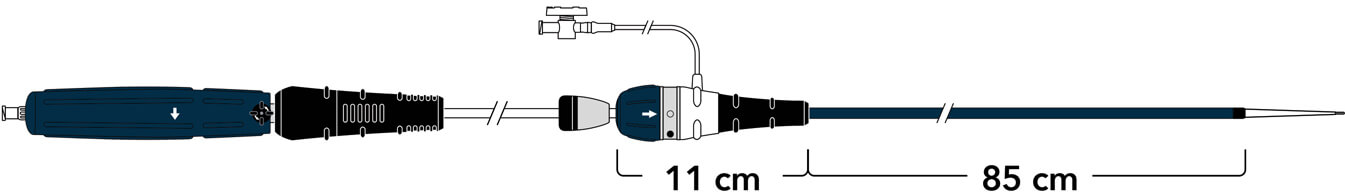
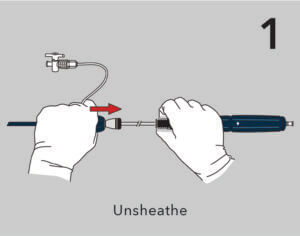
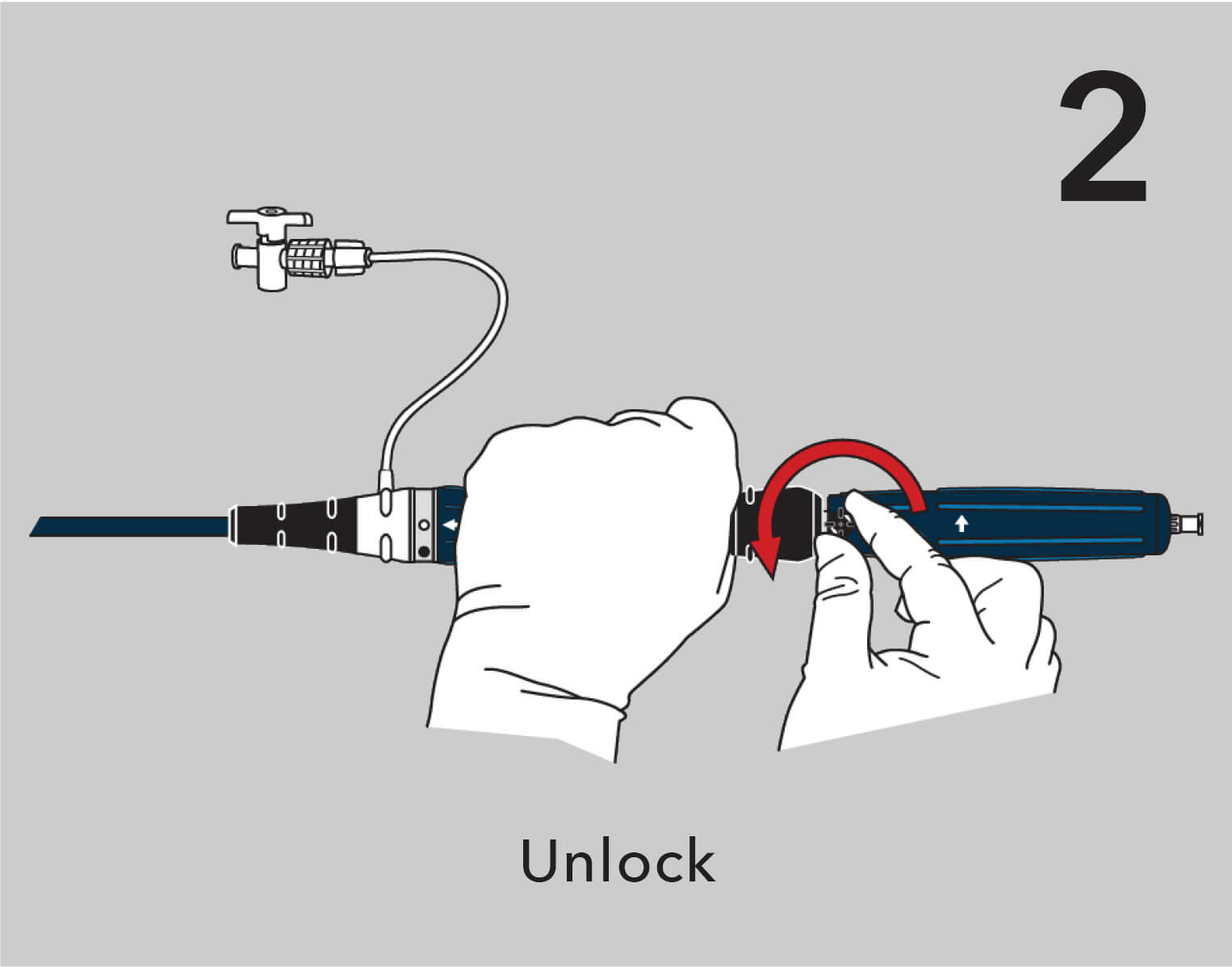
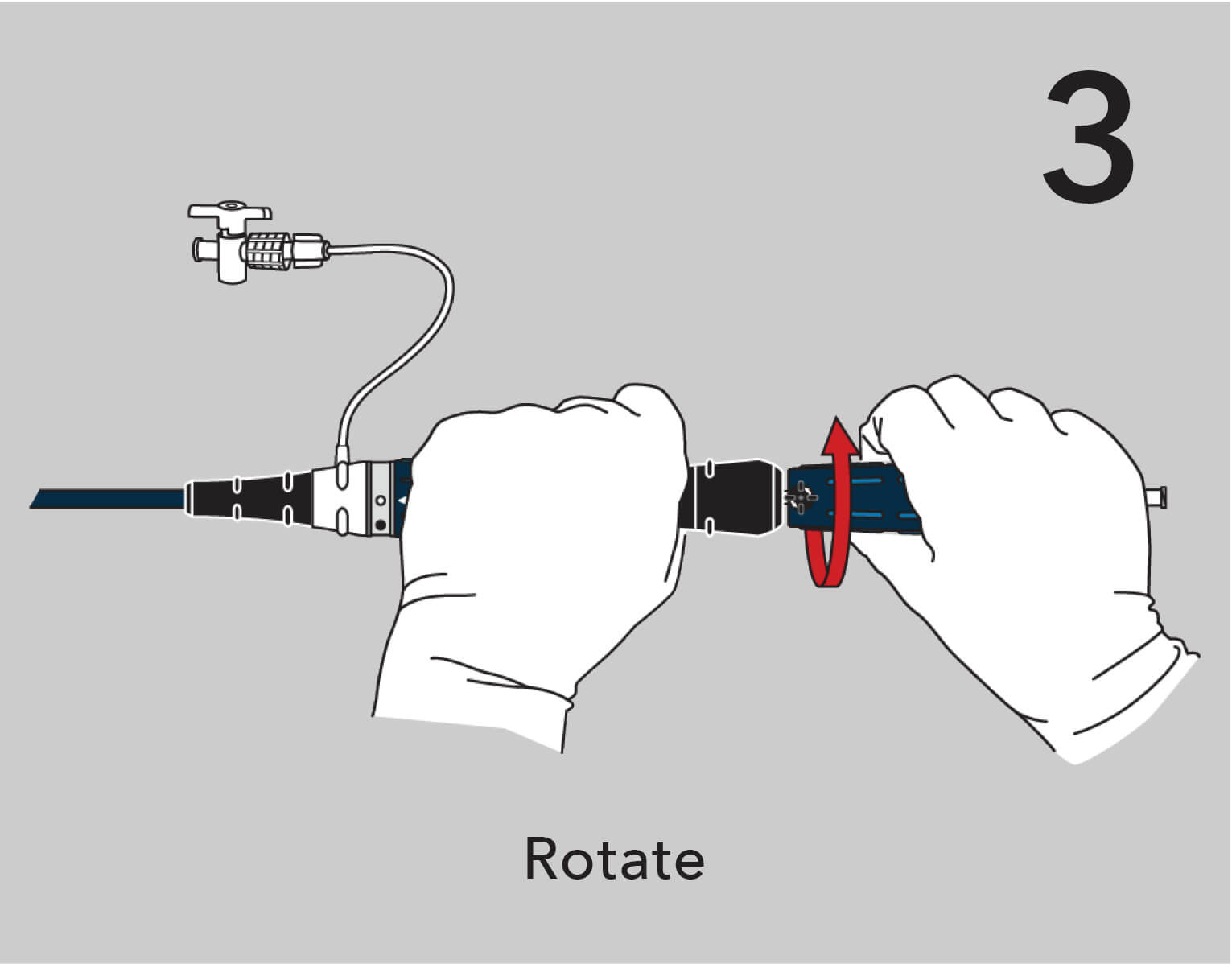
A new ergonomic handle simplifies deployment while maintaining precision and control.

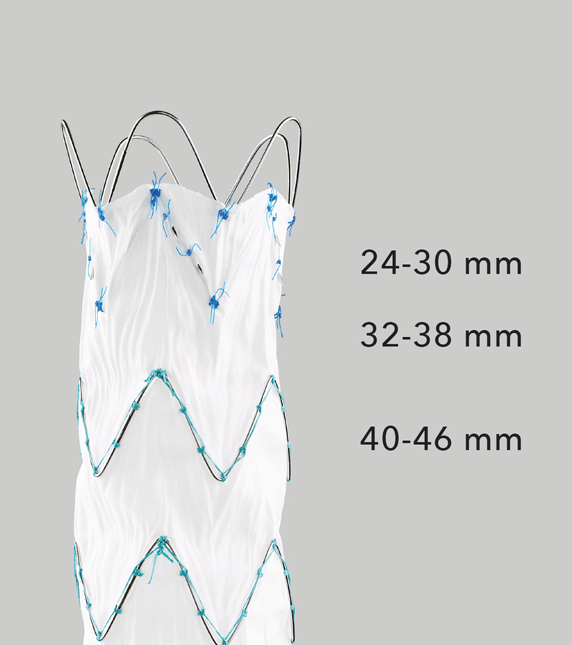
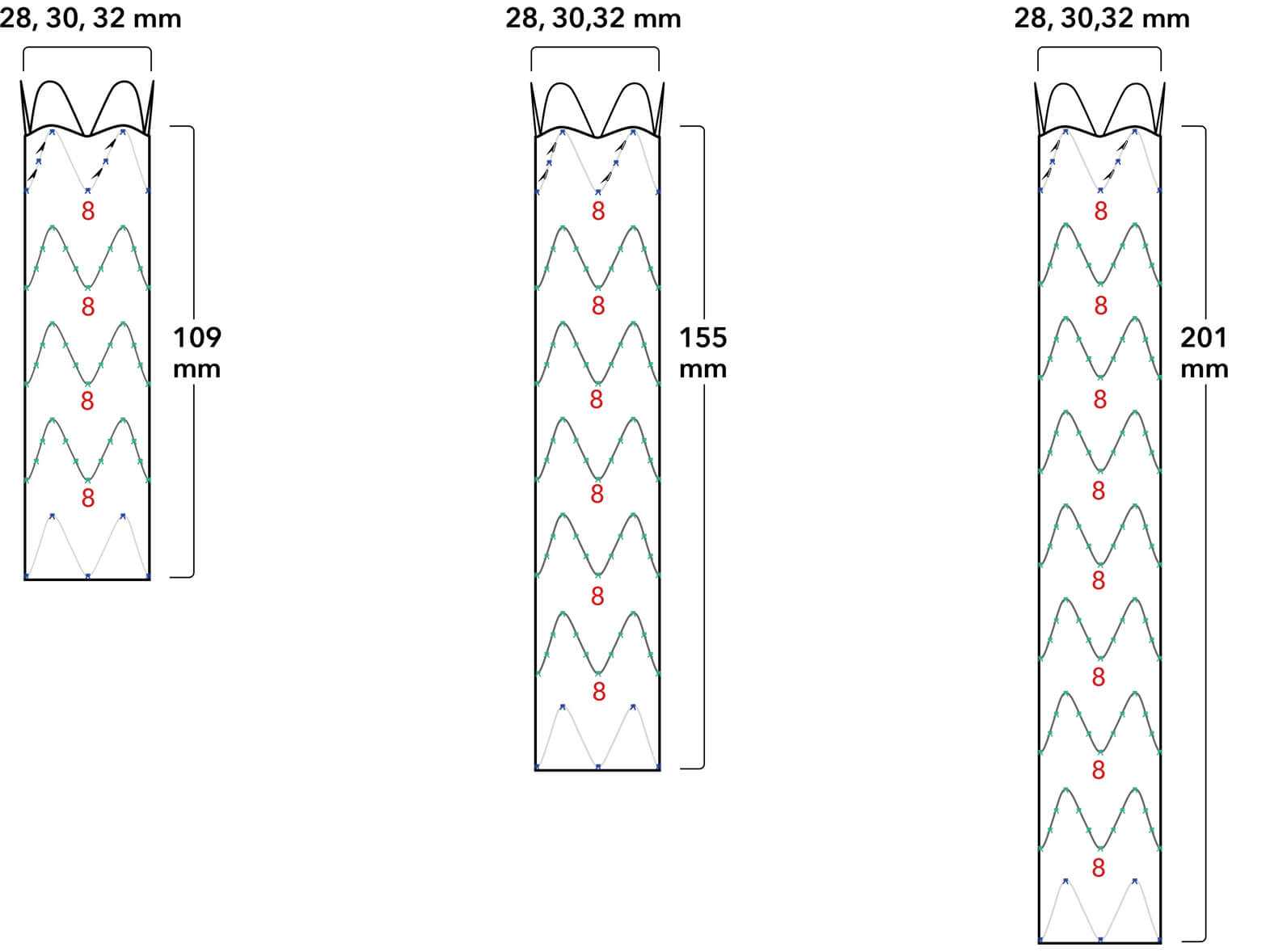
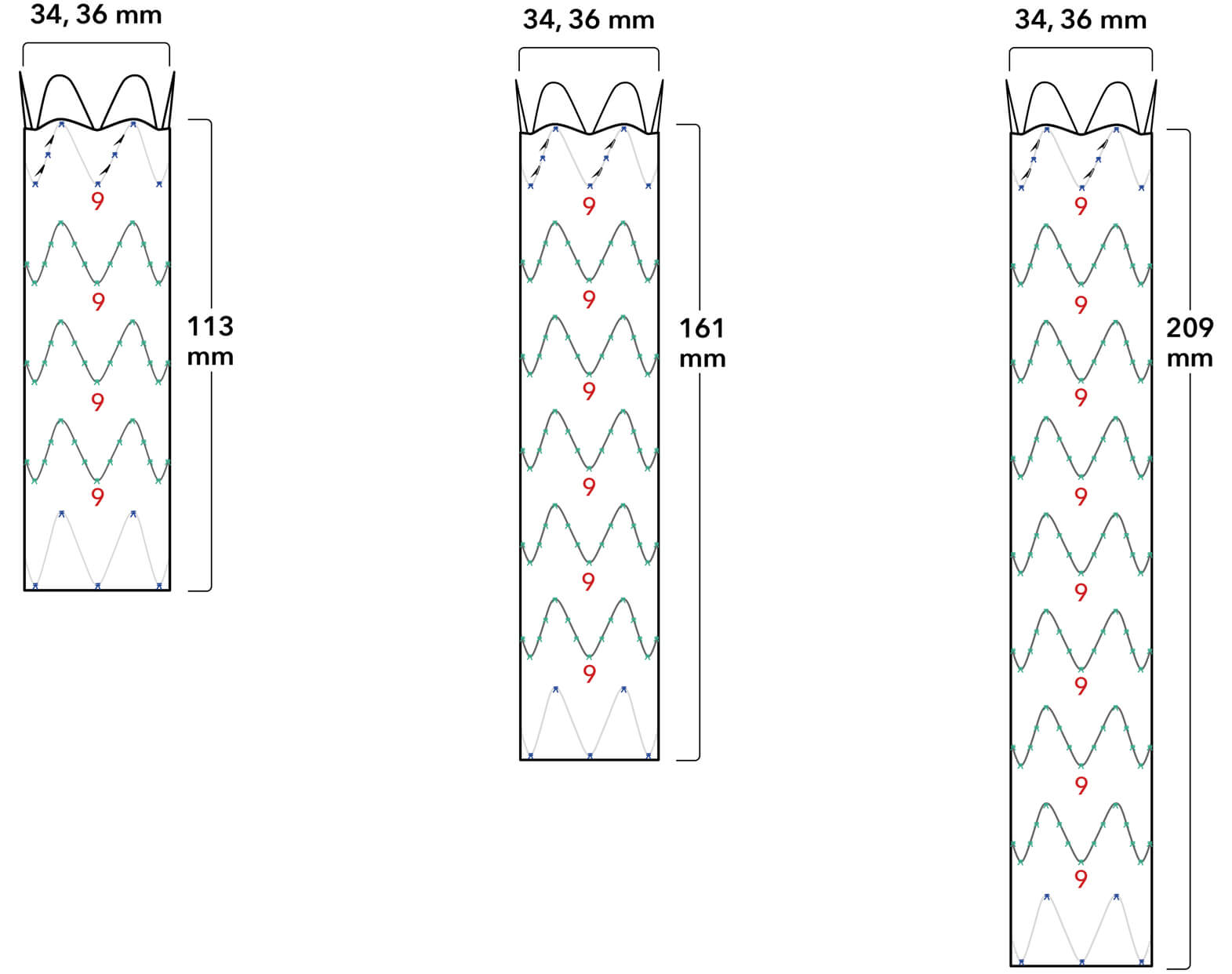
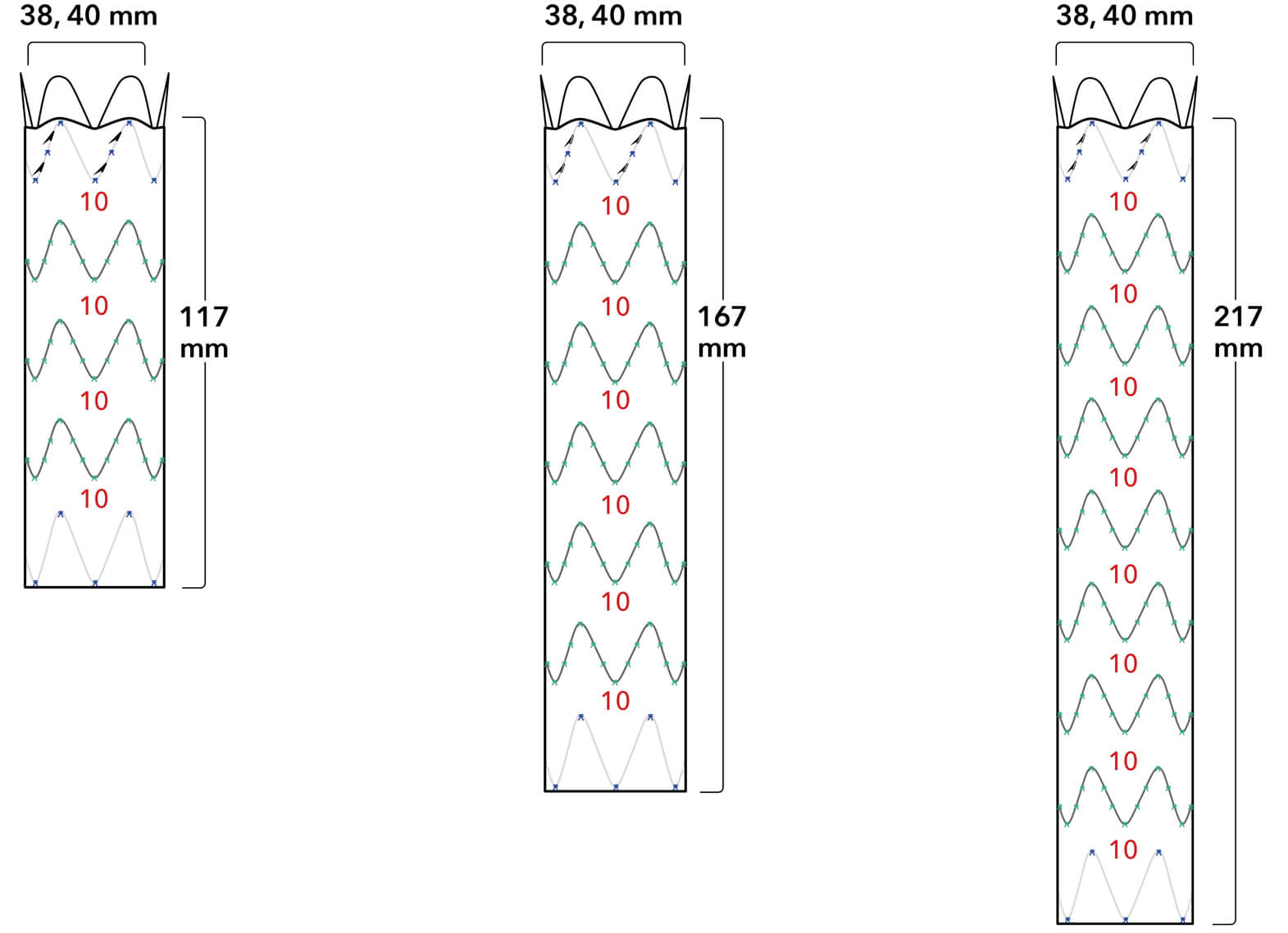
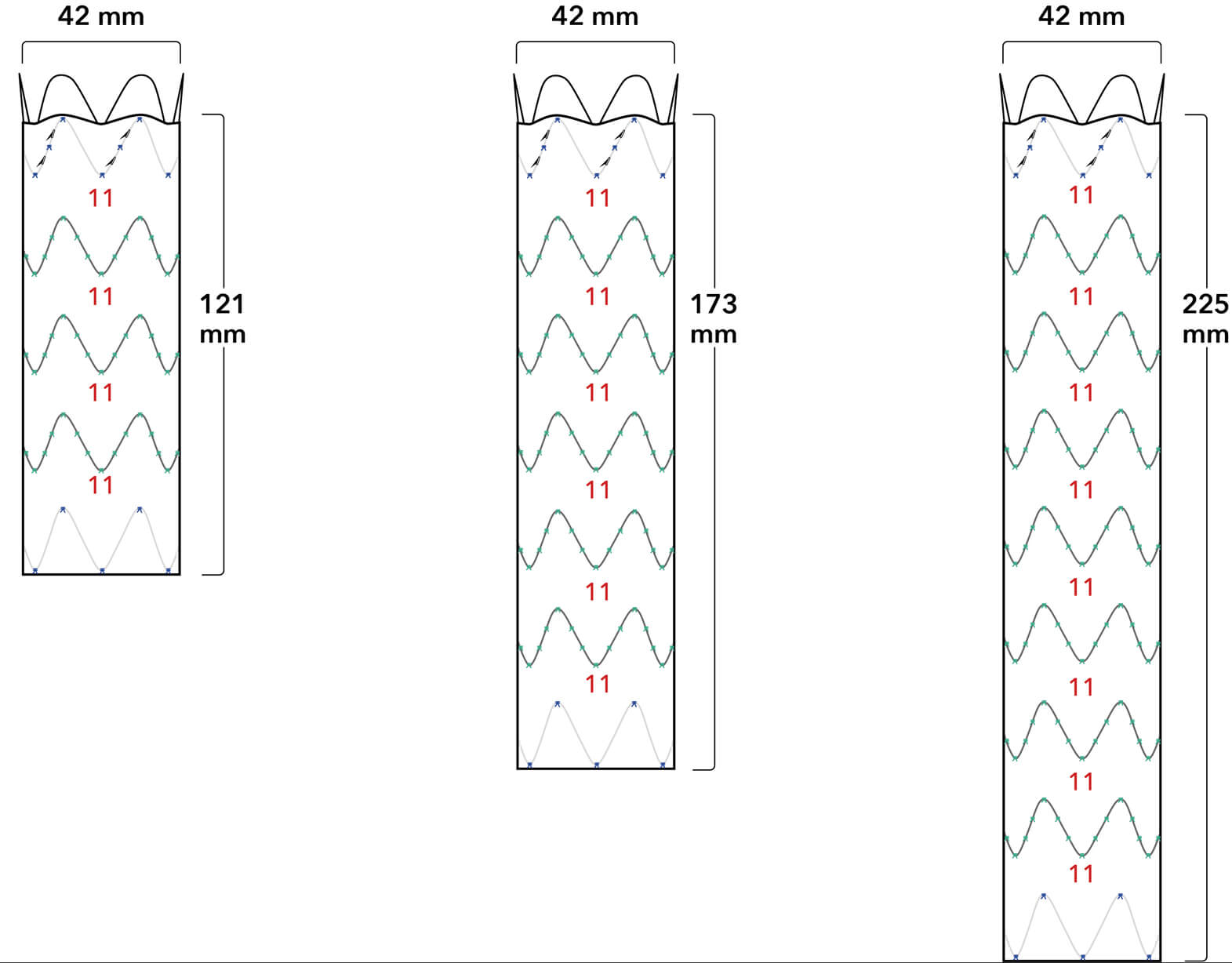
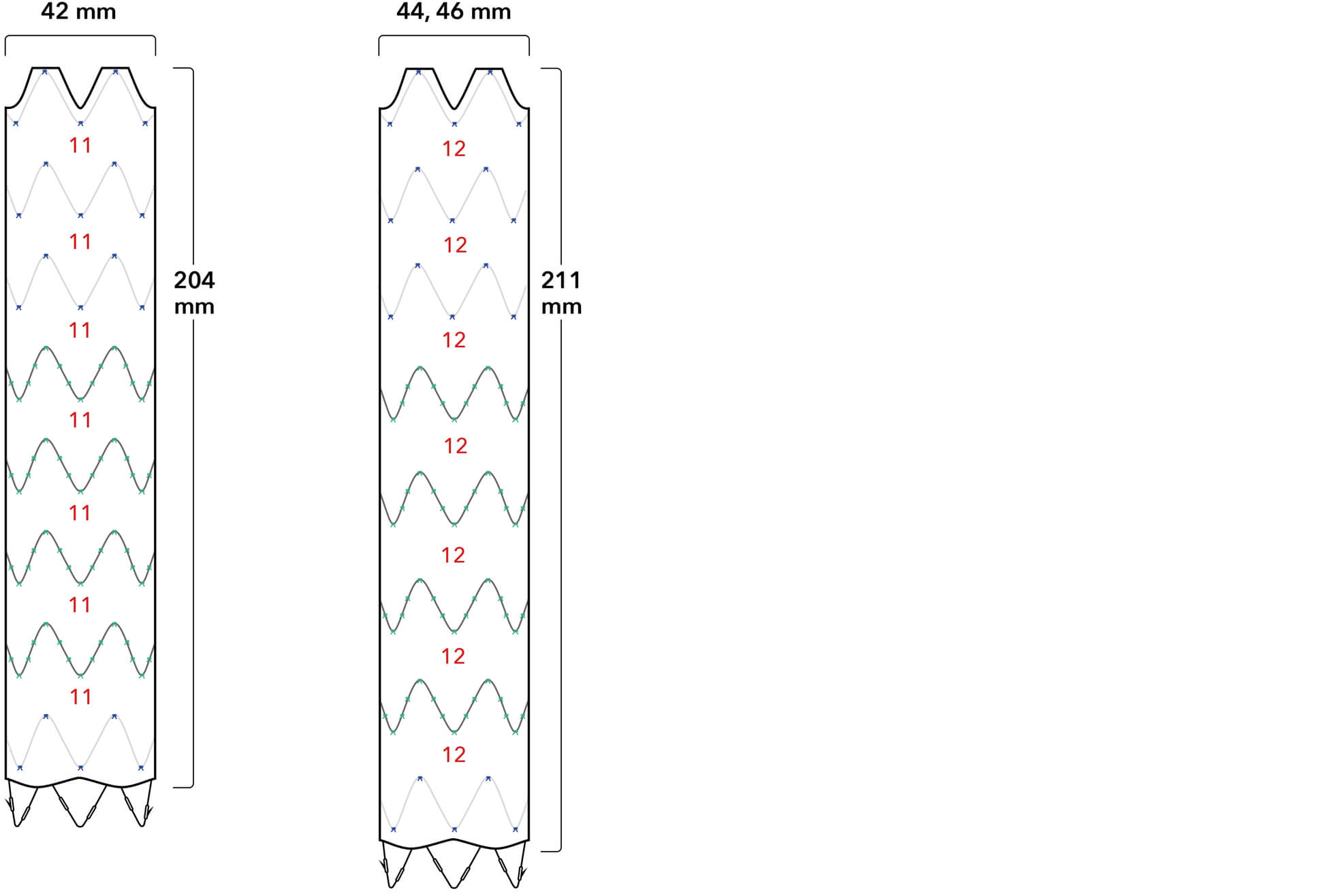
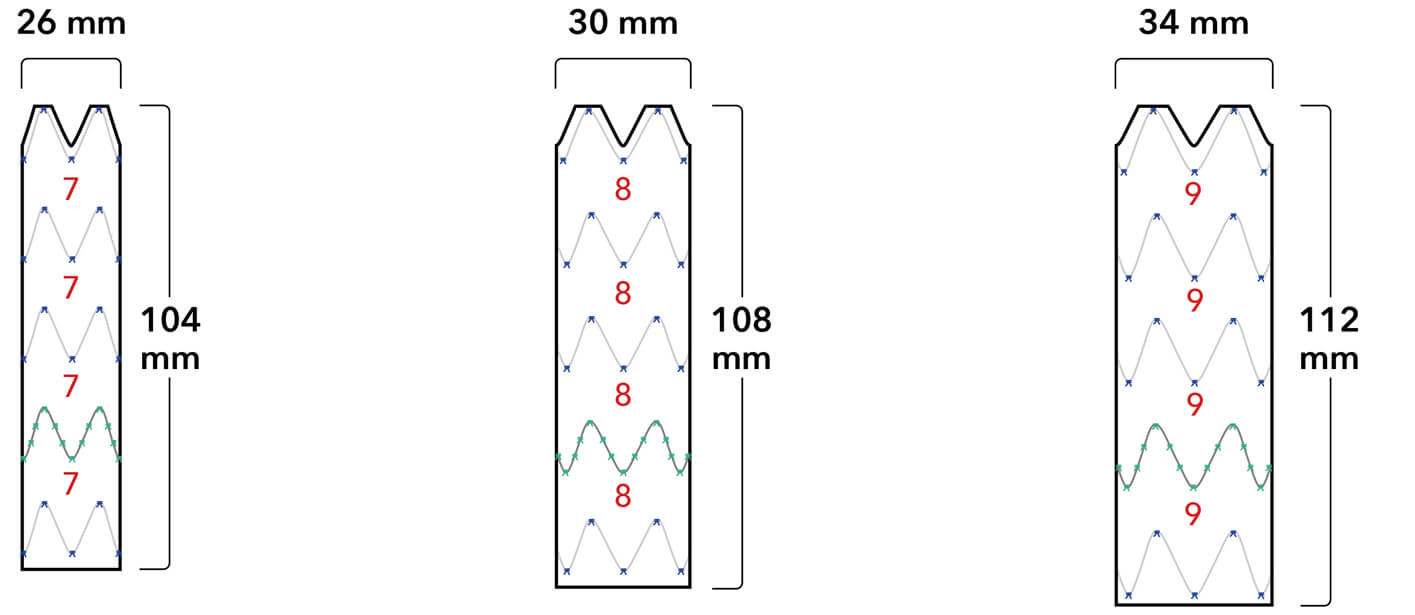
Graft Diameters

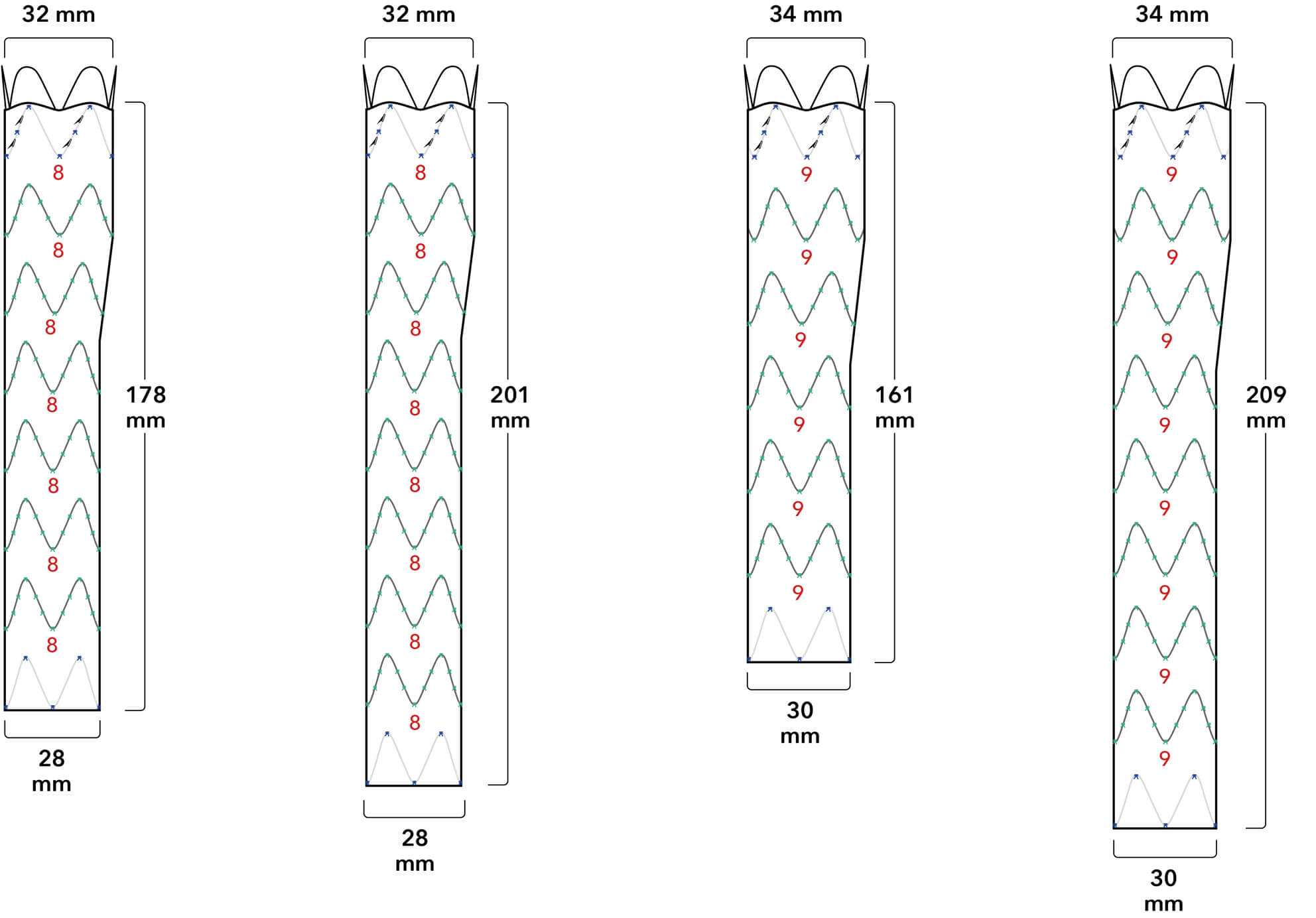
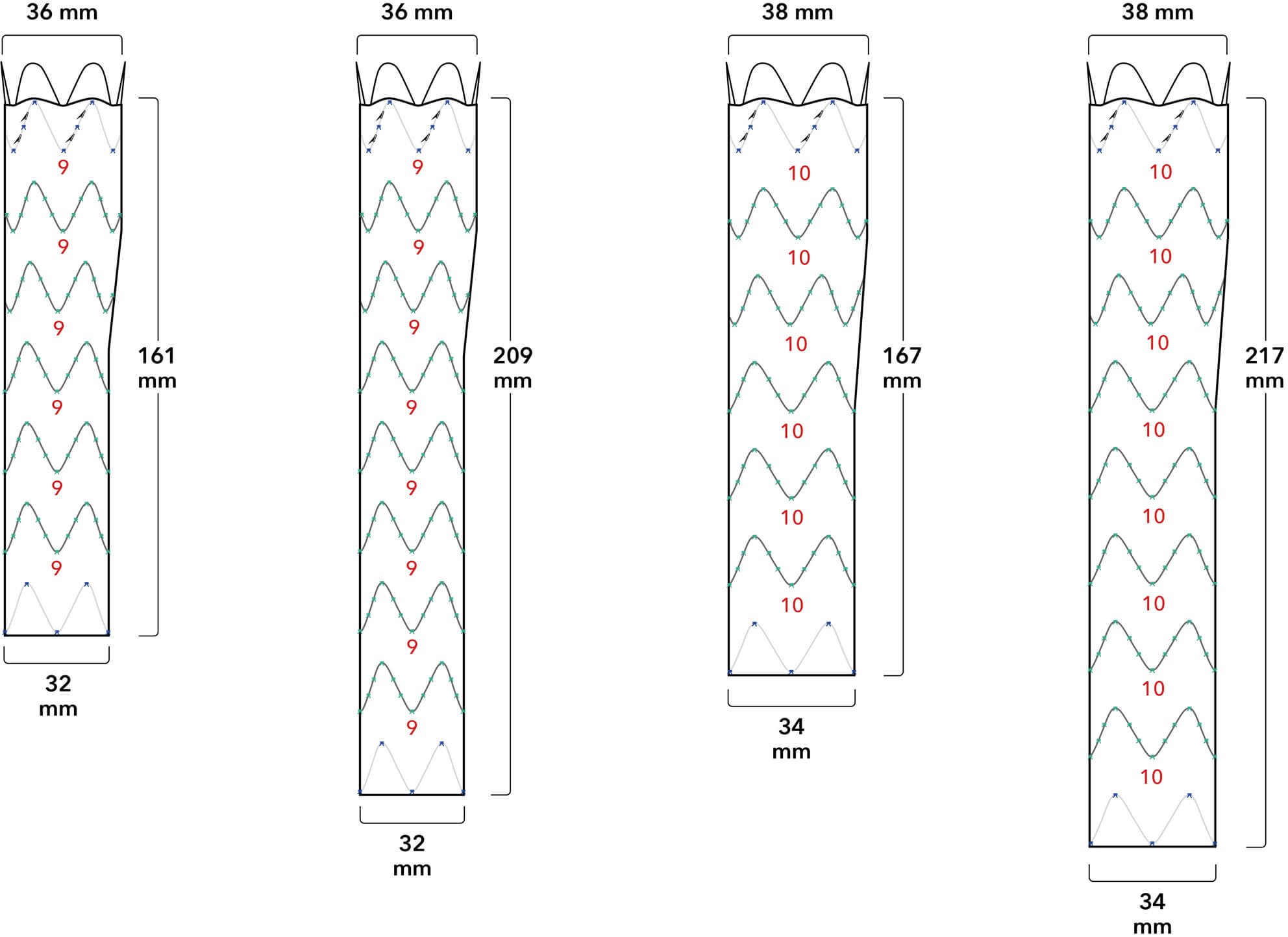
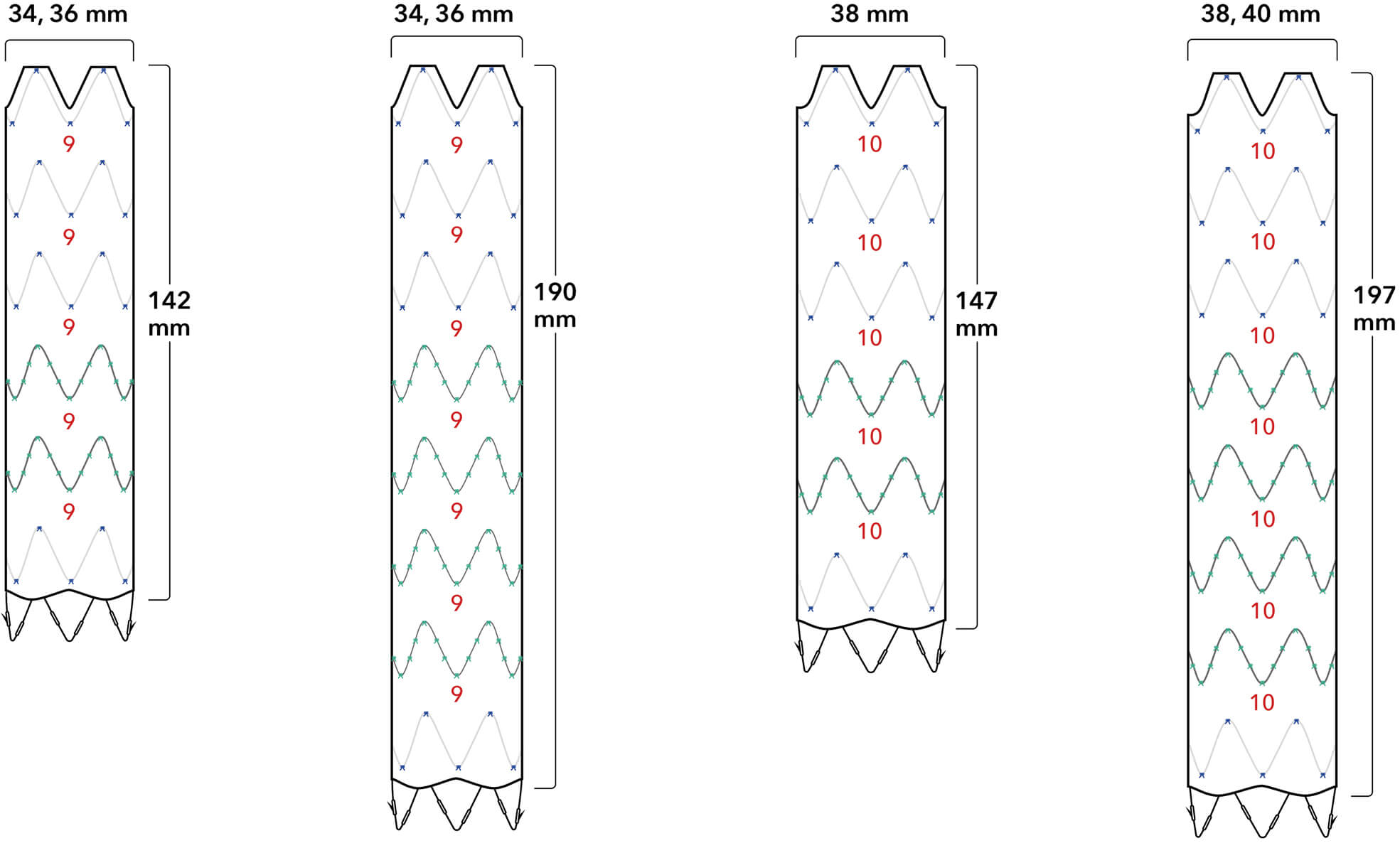
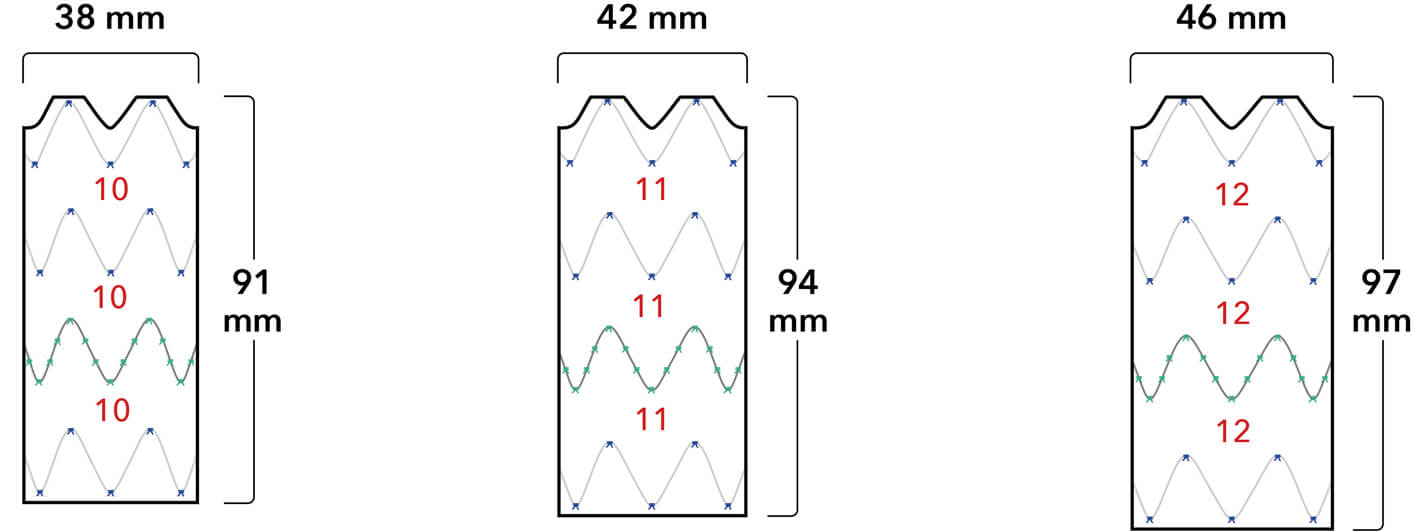
Introducer Sheath Diameters
Proximal conformability helps maintain fixation and seal. A two-piece, modular design enables a patient-specific fit.
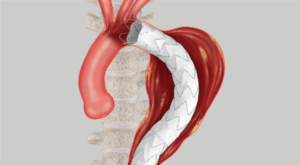
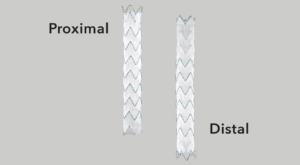
Land the graft exactly where you want it with precise control.
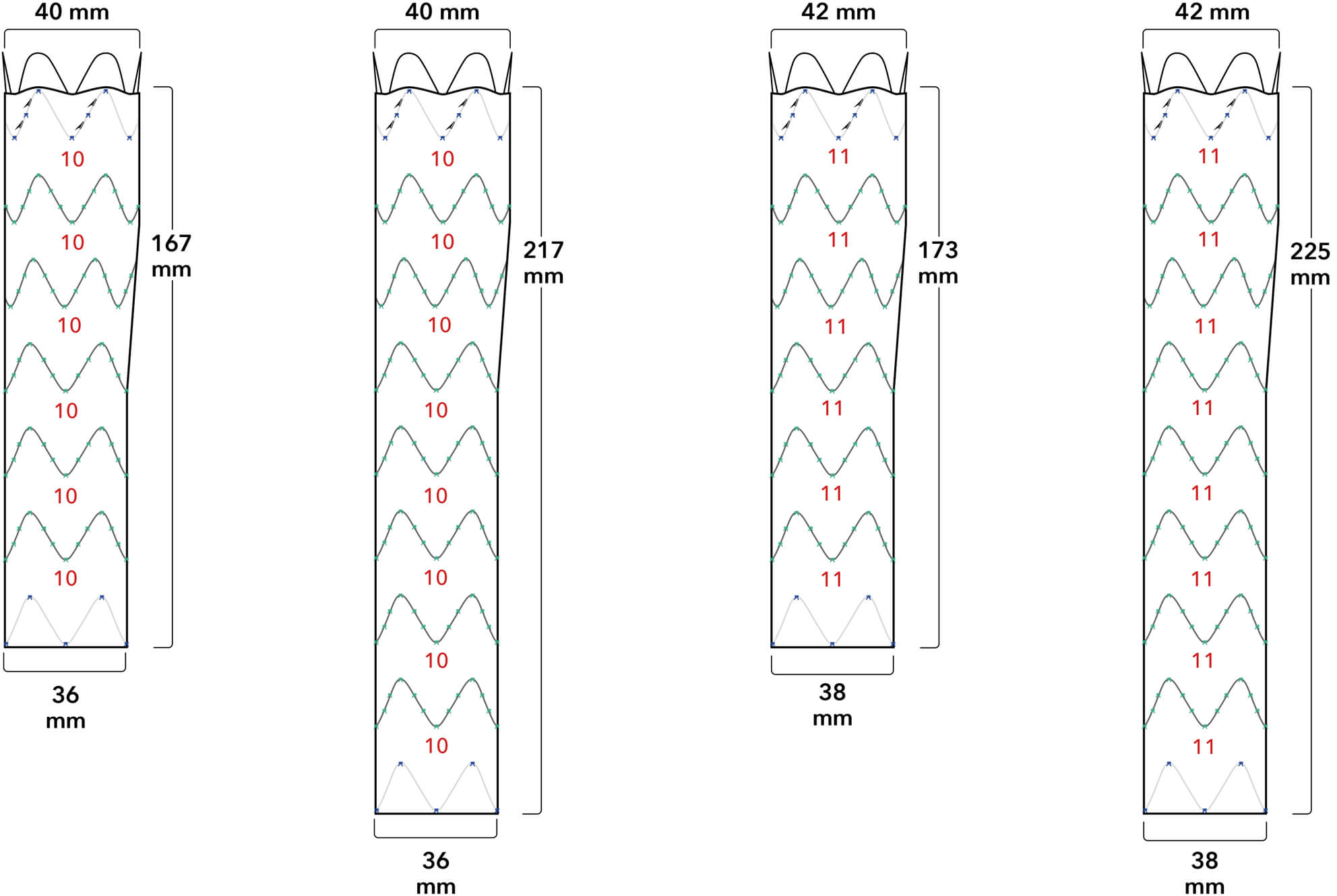
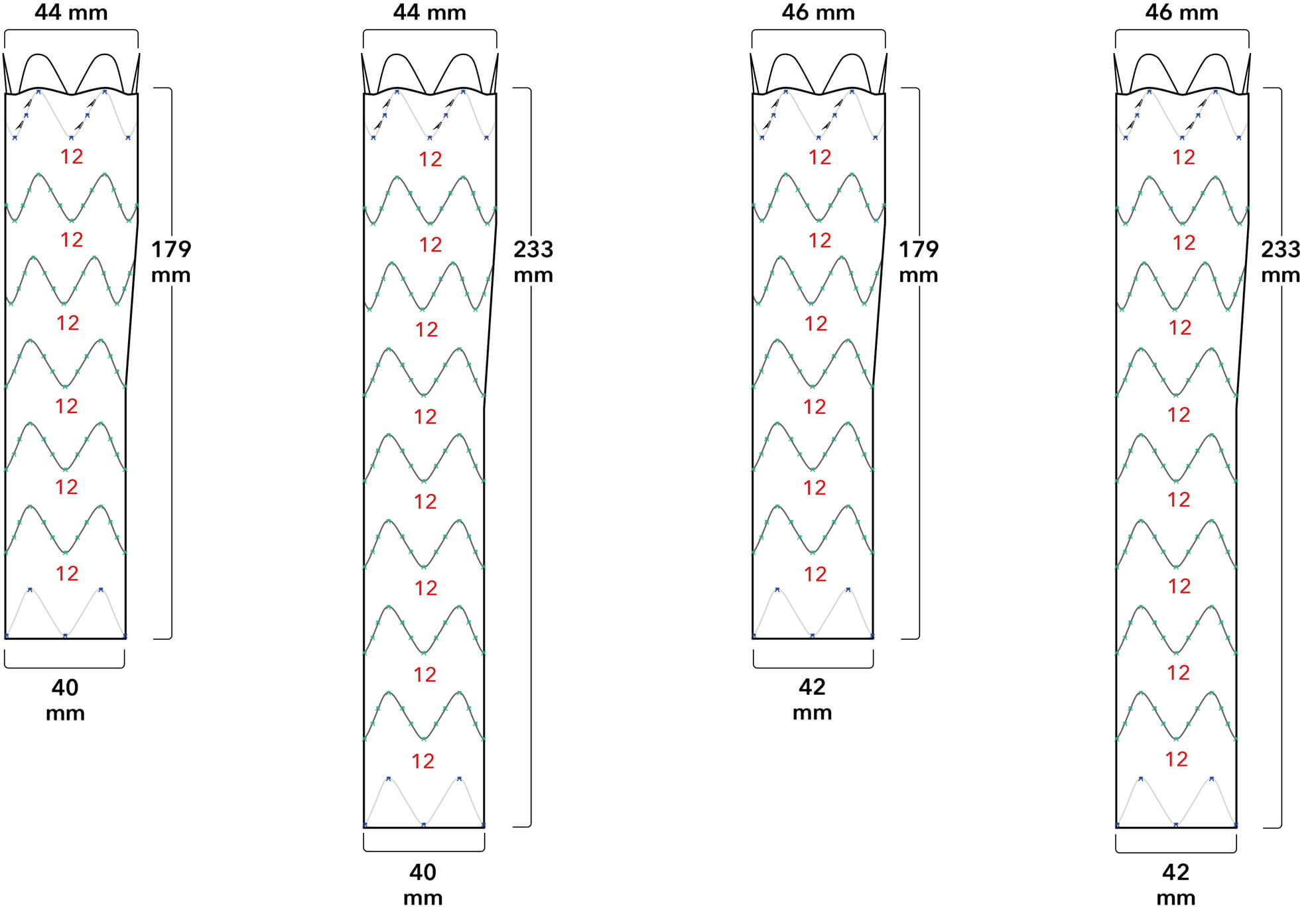
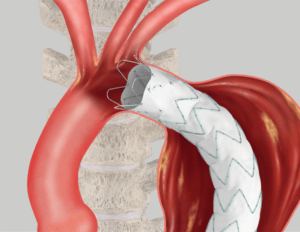 Proximal Fixation
Proximal Fixation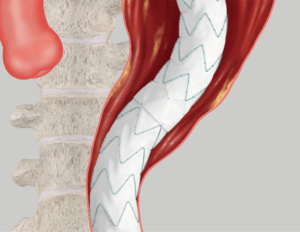 Proximal/Distal Overlap
Proximal/Distal Overlap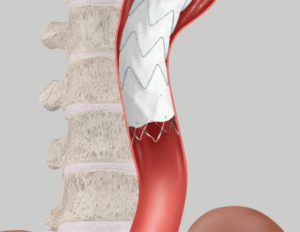 Distal Fixation
Distal Fixation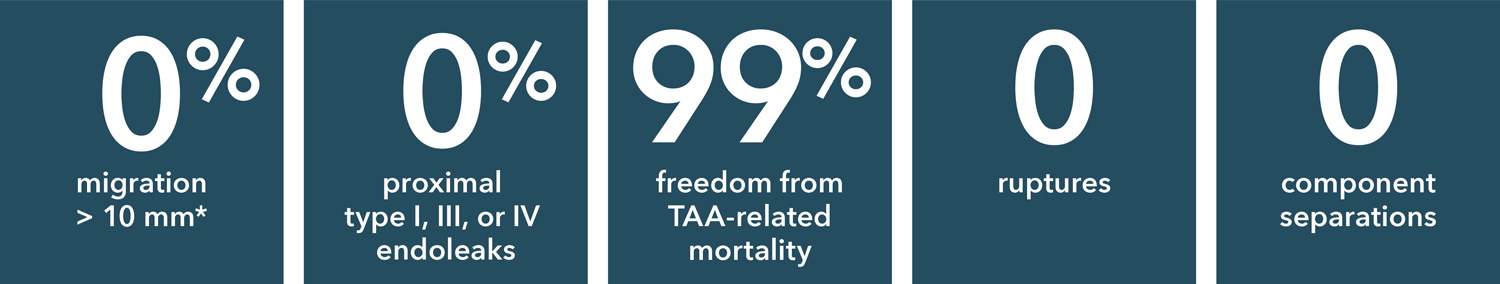
**Based upon new presentation at 5 years. (3 patients had cranial migration of the distal end, first noted at 2 years)
Source: Zenith Alpha Thoracic 2017-2018 clinical update. 110 patients were included in the Global Clinical Study.
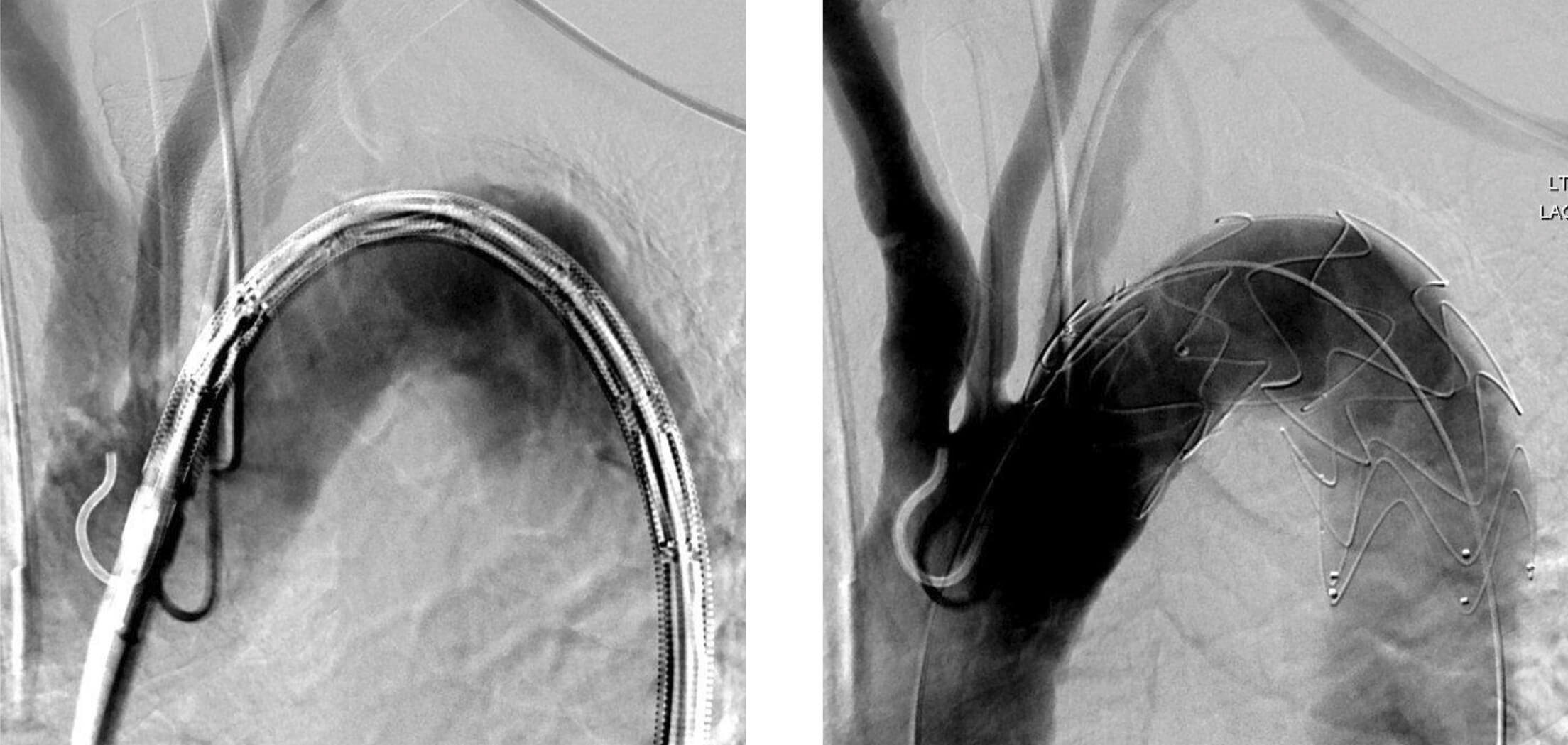
Patient 1: Zenith Alpha Thoracic pre-op/post-op fluoro imaging.
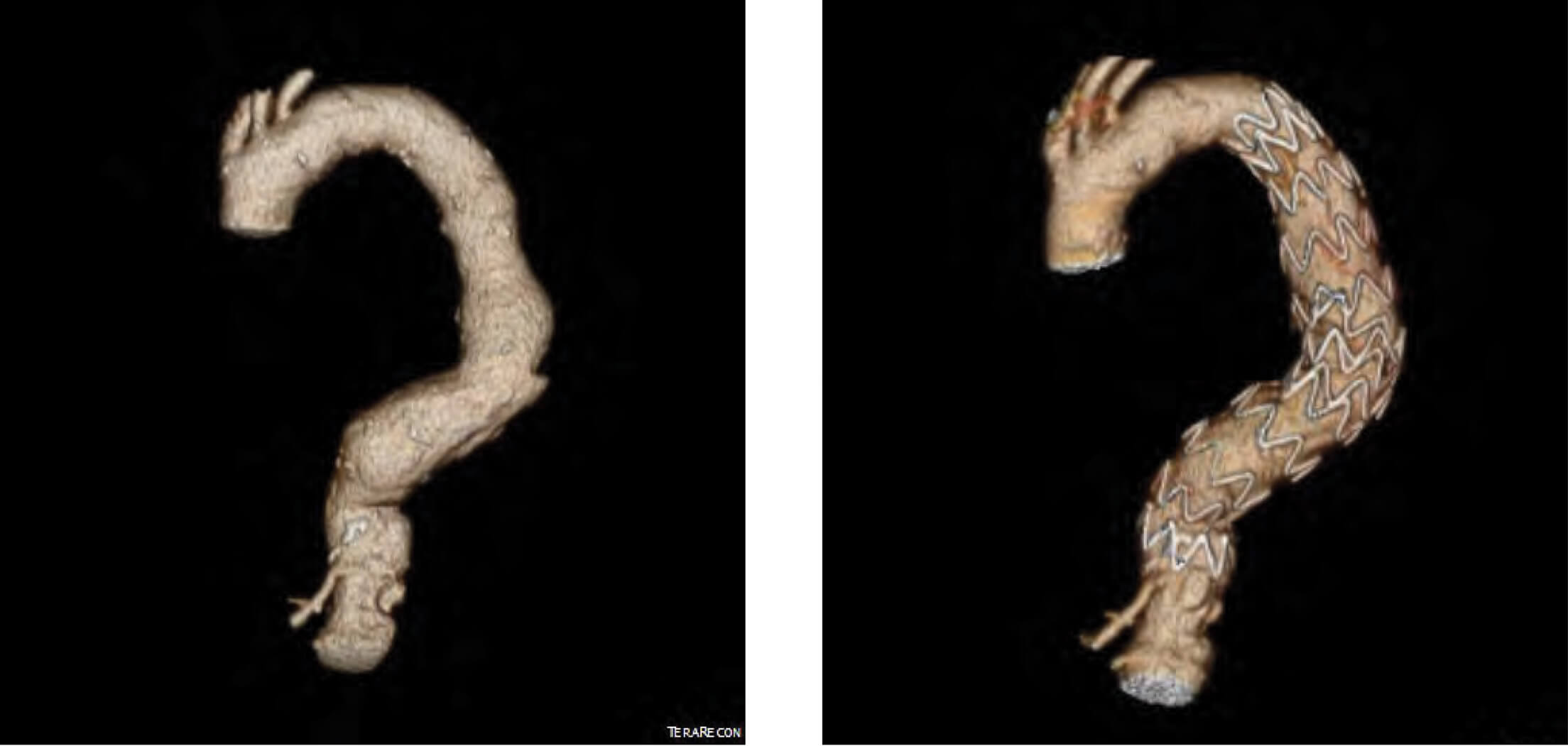
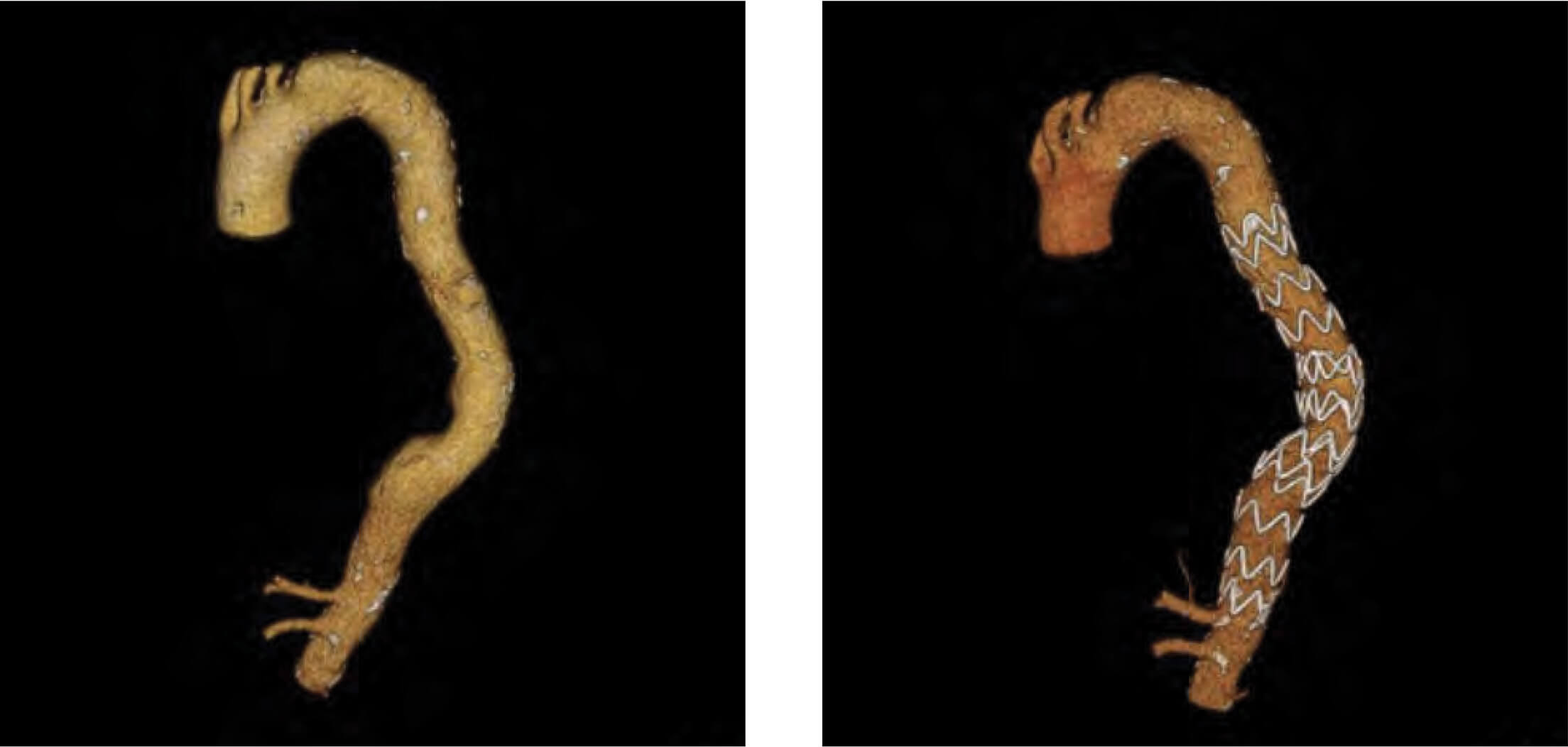
Patient 2: Zenith Alpha Thoracic pre-op/post-op TeraRecon imaging.
Patient 3: Zenith Alpha Thoracic pre-op/post-op TeraRecon imaging.
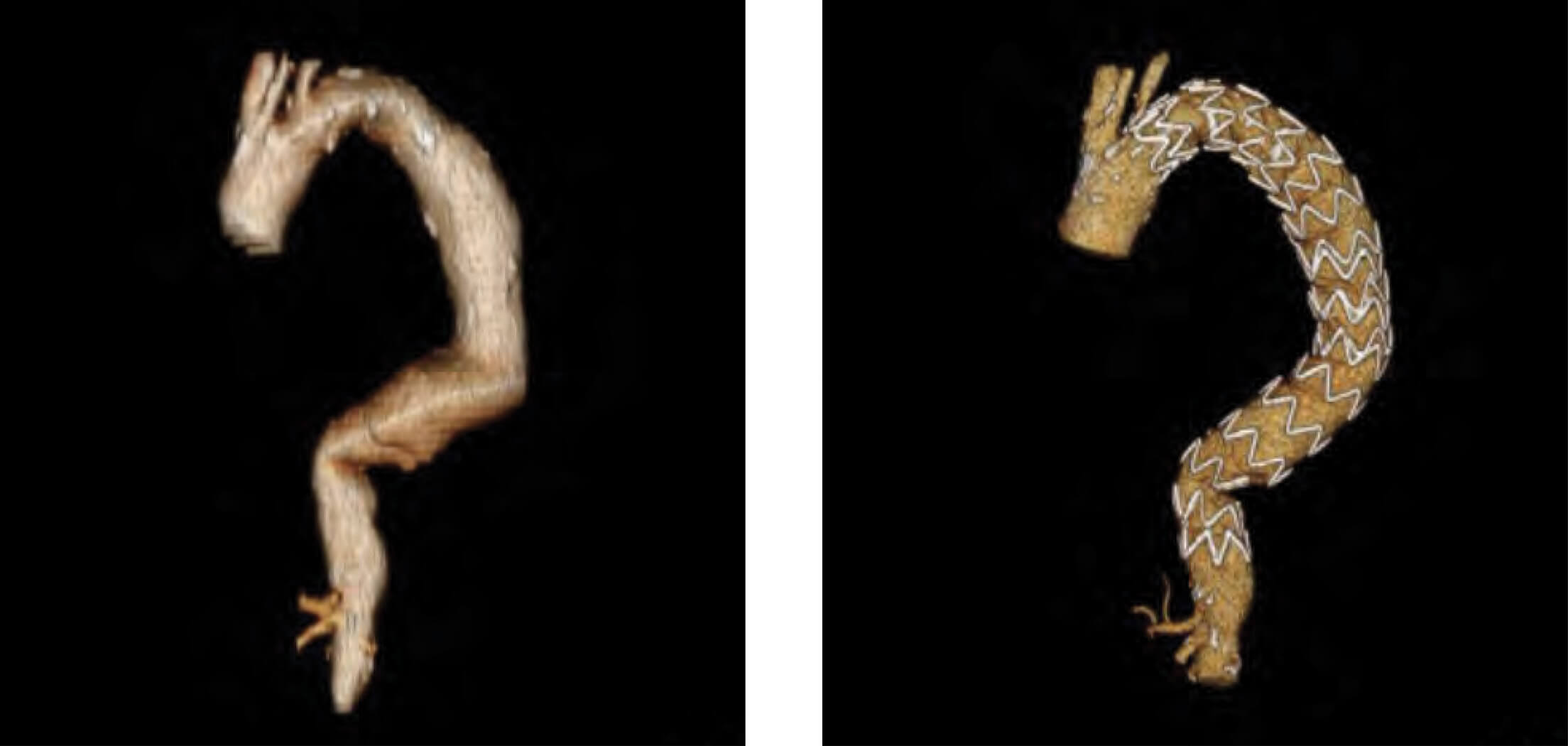
Patient 4: Zenith Alpha Thoracic pre-op/post-op TeraRecon imaging.
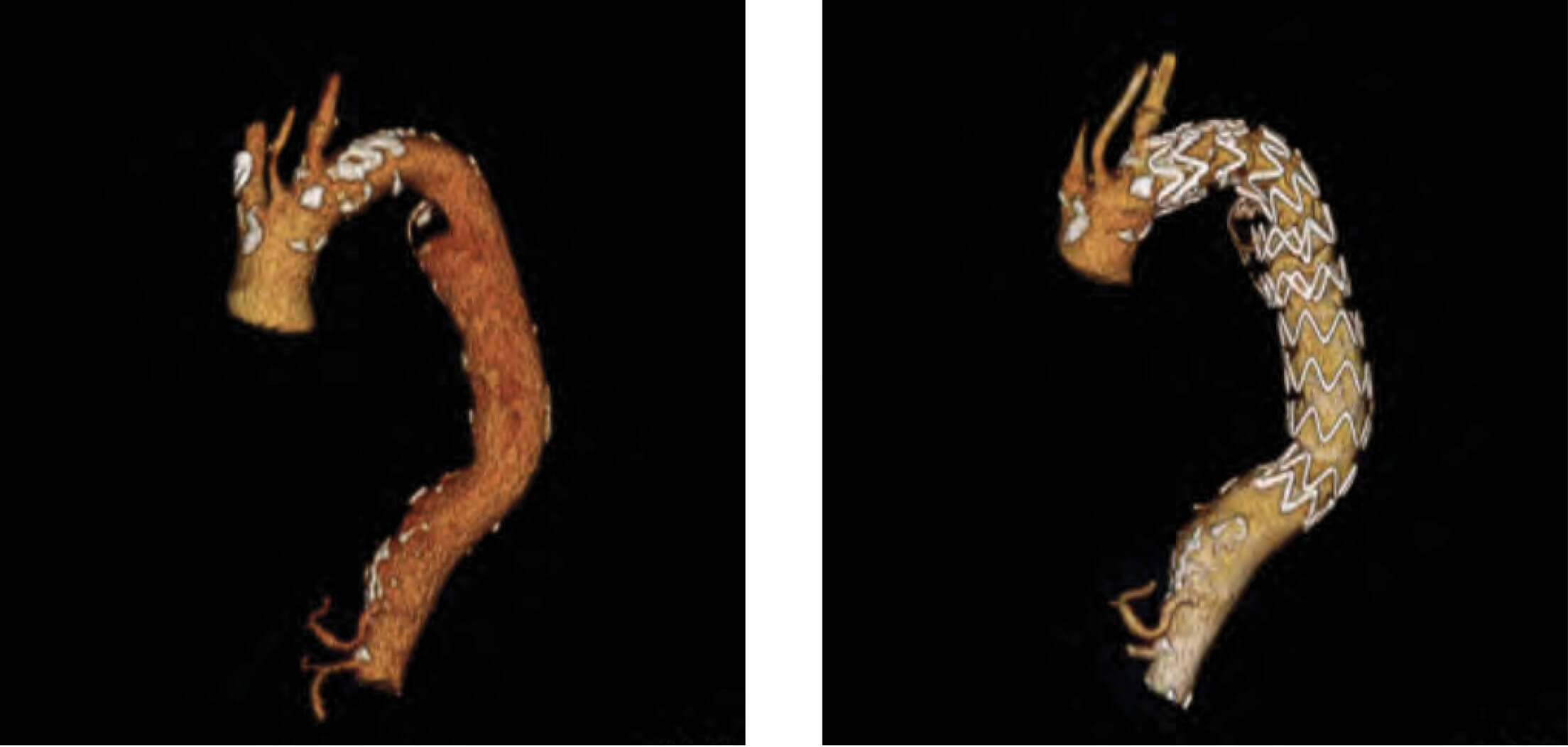
Patient 5: Zenith Alpha Thoracic pre-op/post-op TeraRecon imaging.