Covering the entry tear may not be enough. Address the specific challenges involved in tr eating type B dissection.
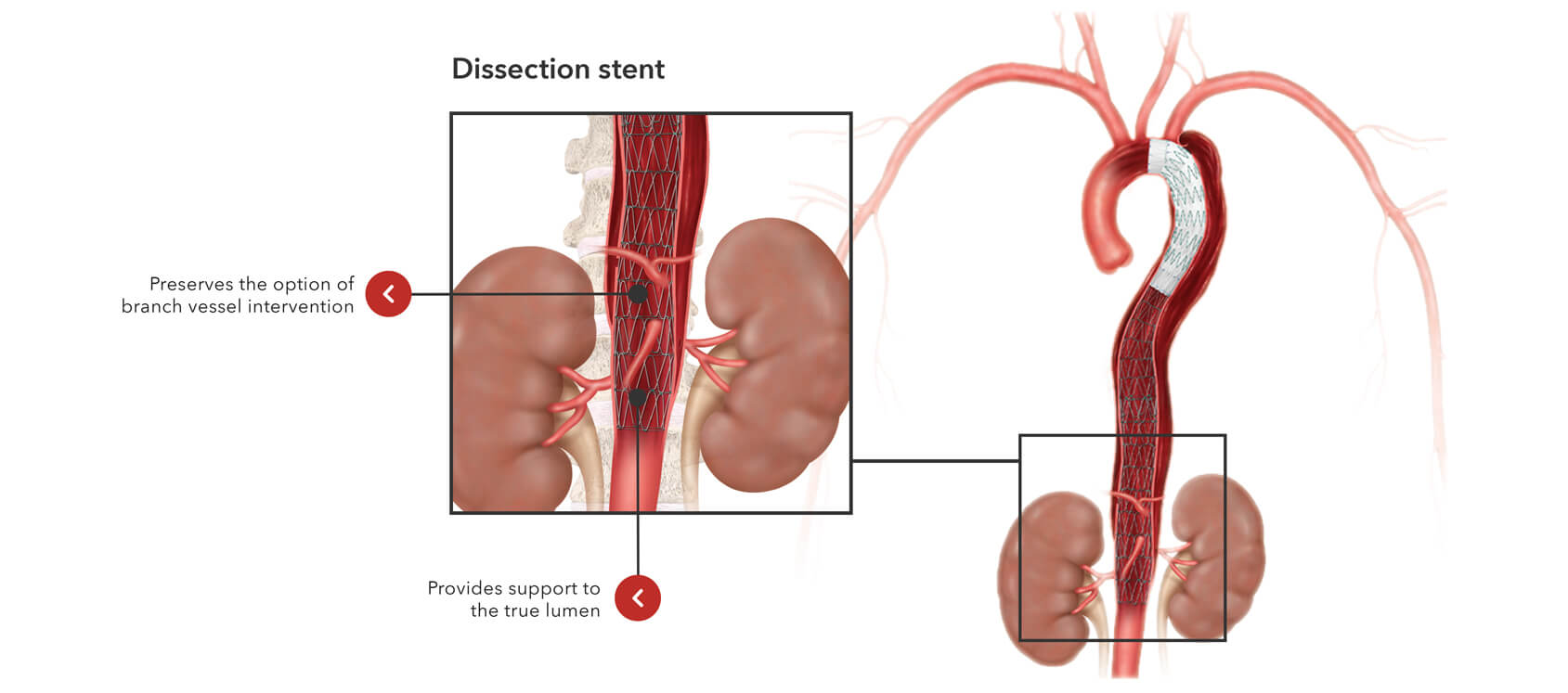
Designed to minimize graft- and procedure-induced trauma and complications.
No barbs or proximal bare stent
Atraumatic to help reduce the risk of retrograde dissection
11,000+ devices implanted globally
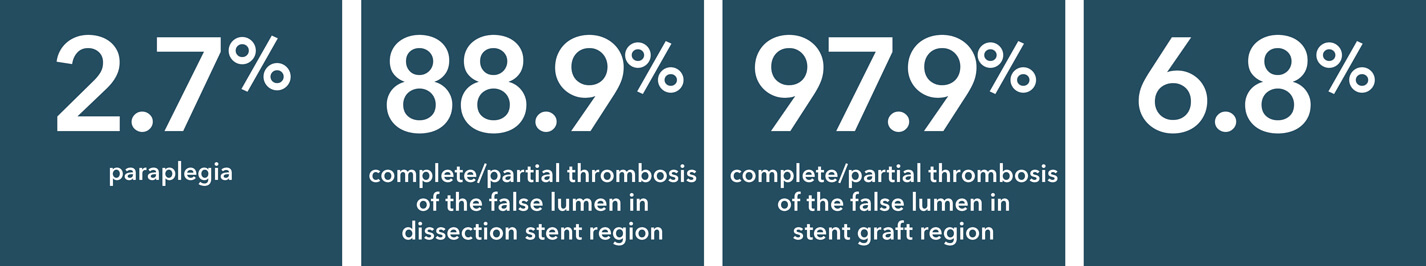
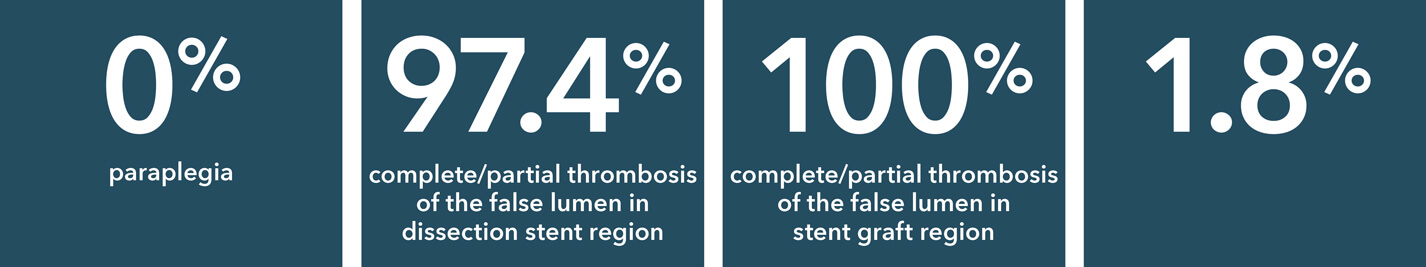
Source: Summary of Clinical Data for IFU 441-01EN (Zenith Dissection Endovascular System). 73 patients were included in the Global Clinical Study.
The complexity of the disease means there can be great pathological variations with each patient.
The support to help you choose the right treatment option at each stage of the disease
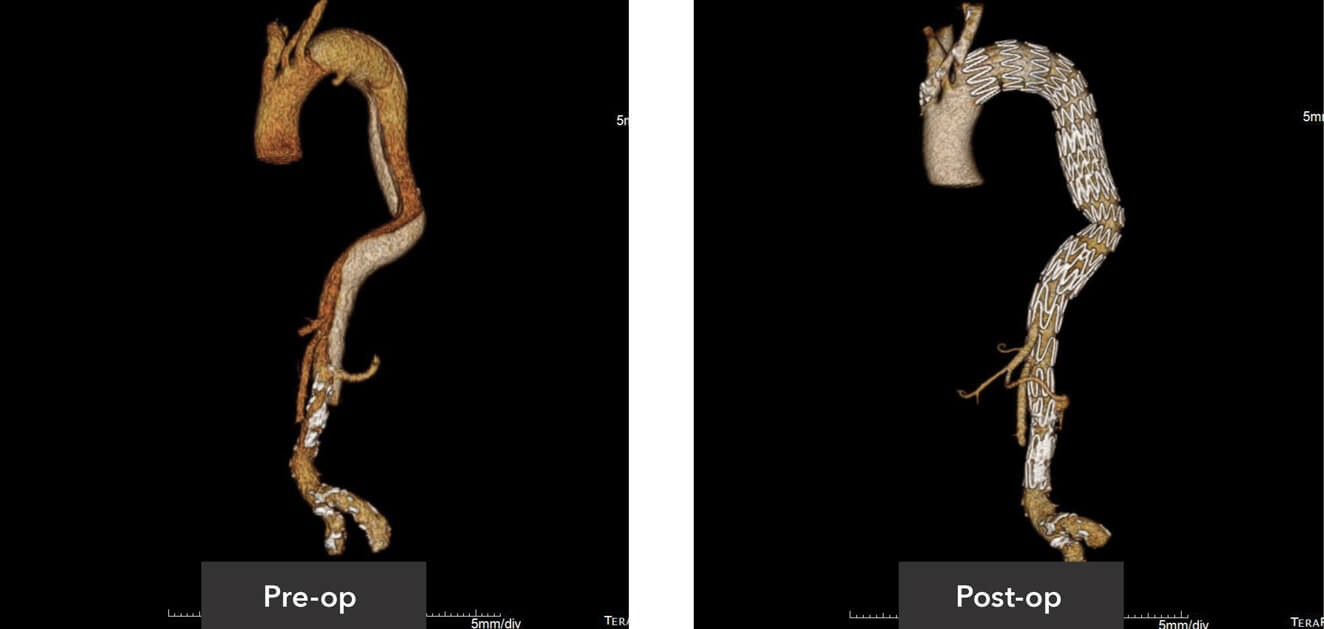
Pre-op/post-op Tera Recon imaging
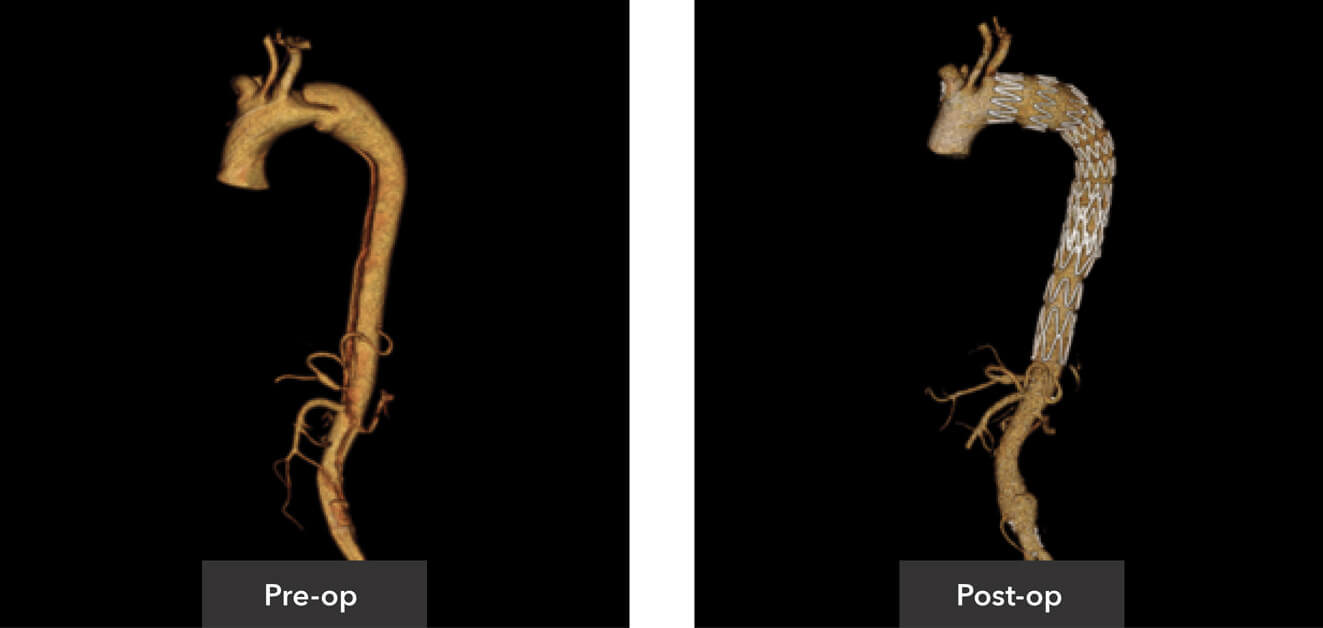
Sagittal view pre-op/post-op CT imaging
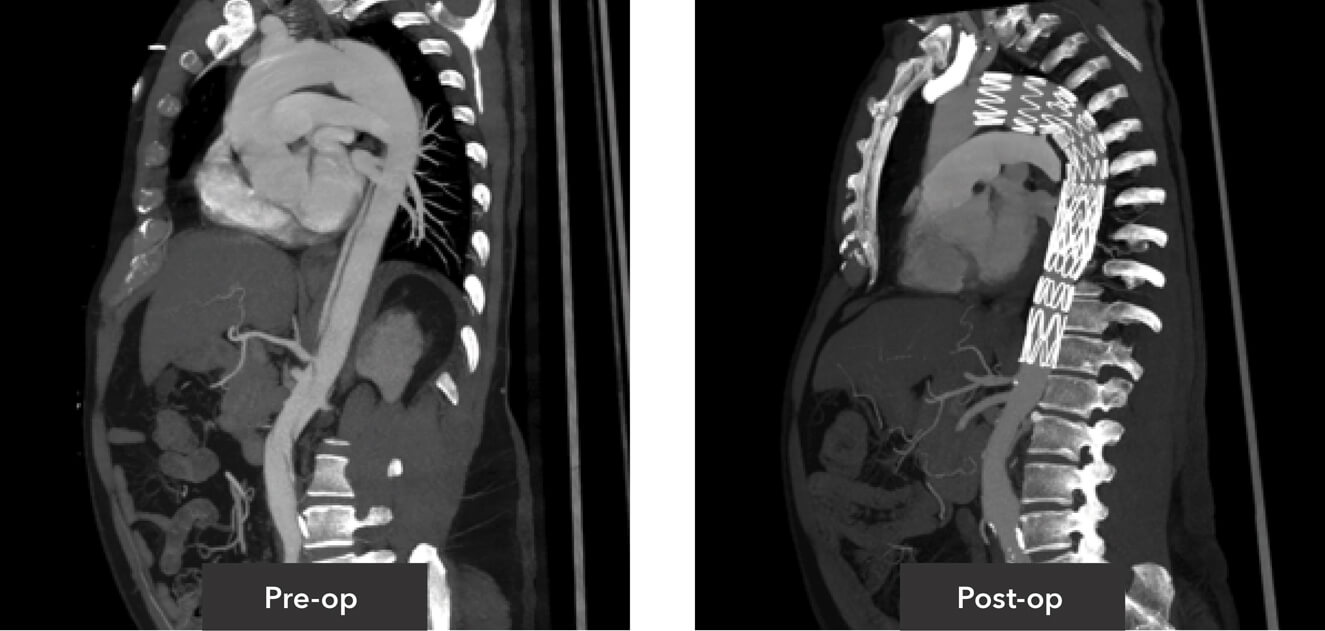
Pre-op/post-op Tera Recon imaging
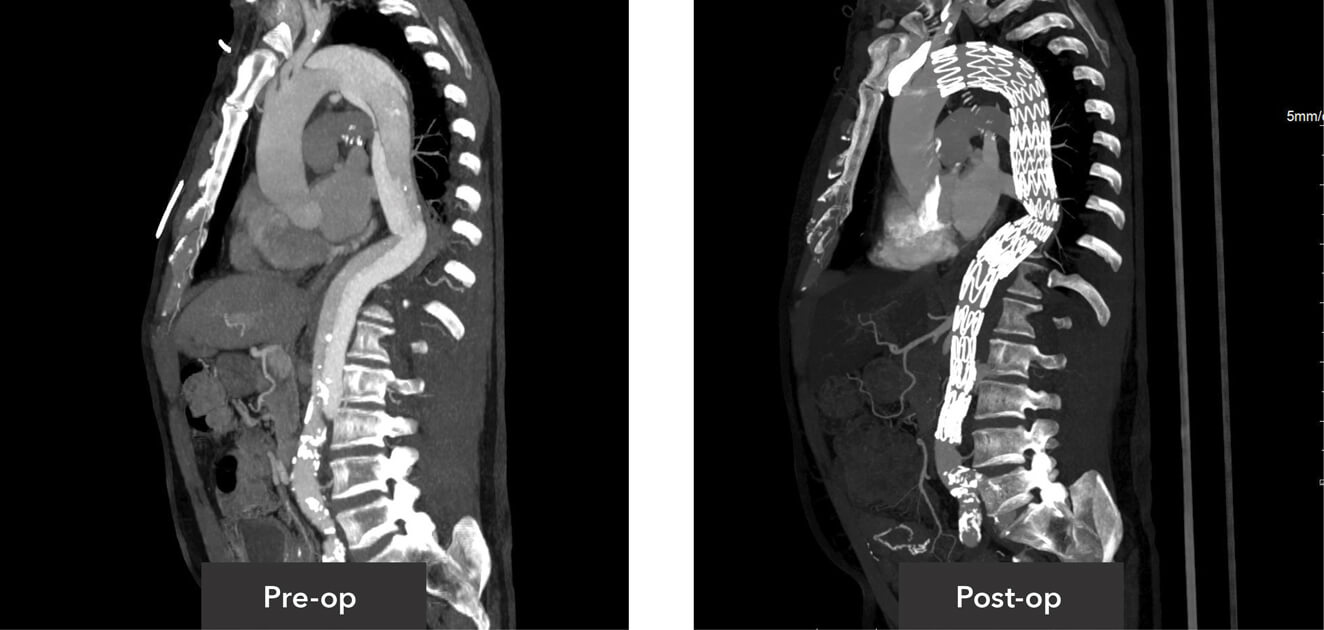
Sagittal view pre-op/post-op CT imaging
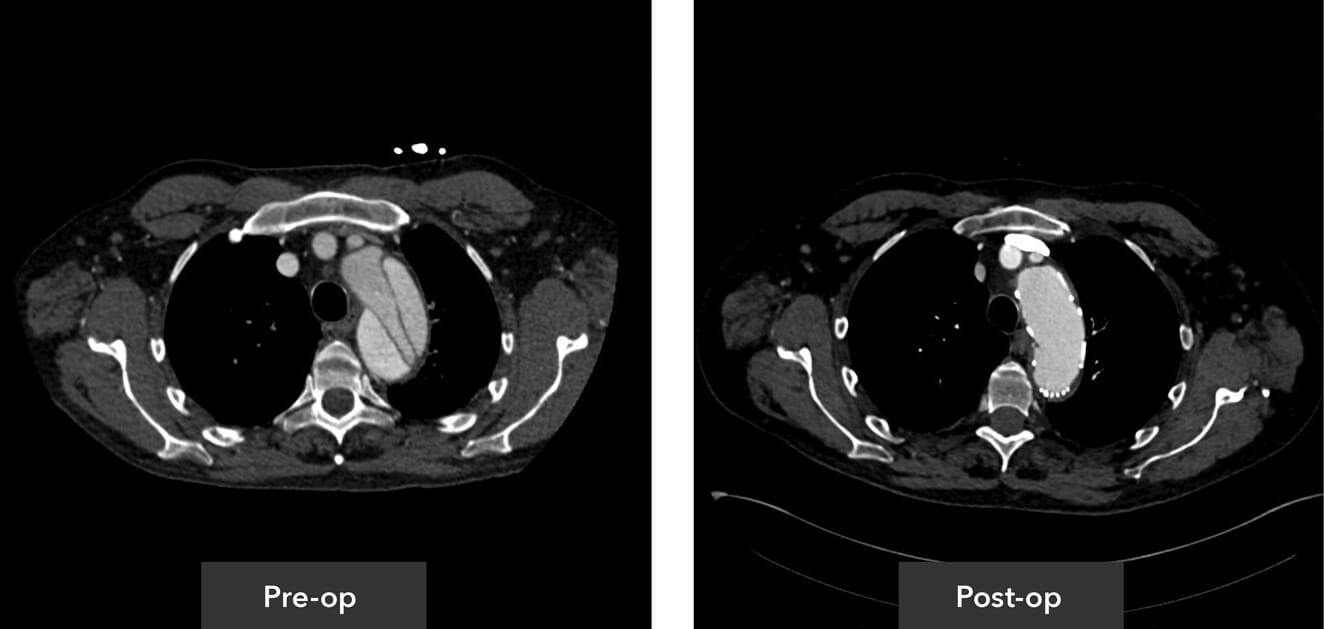
Aortic root view pre-op/post-op axial view CT imaging
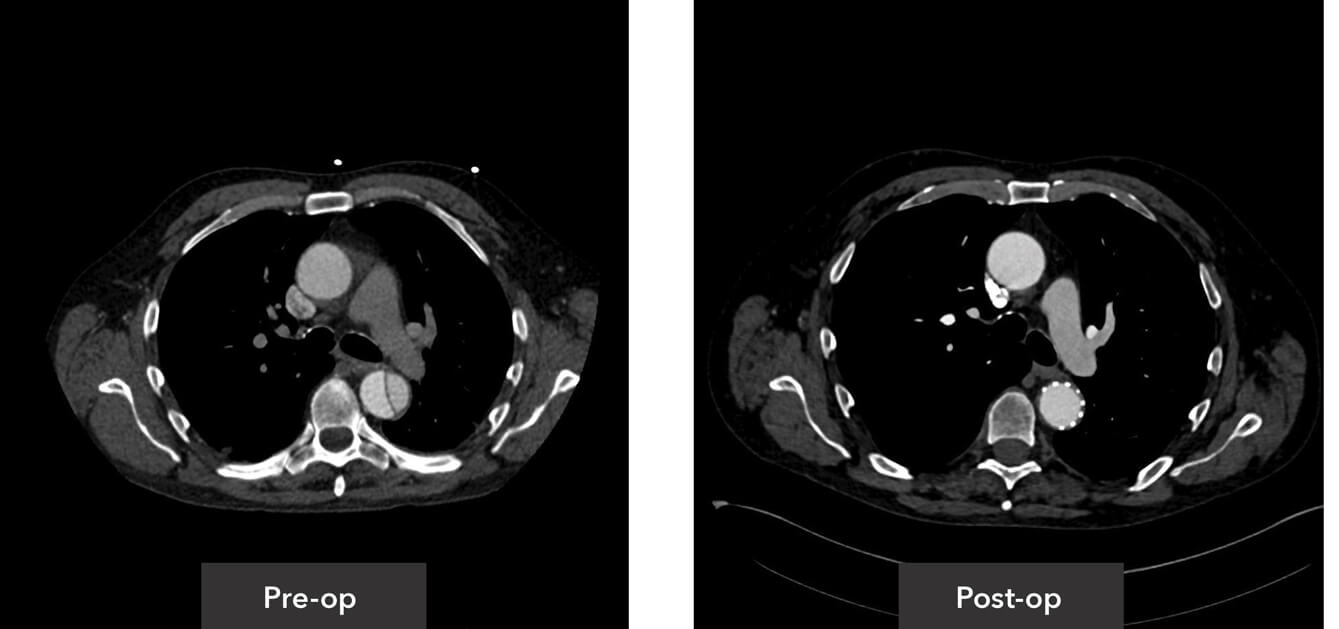
Descending thoracic view pre-op/post-op axial view CT imaging
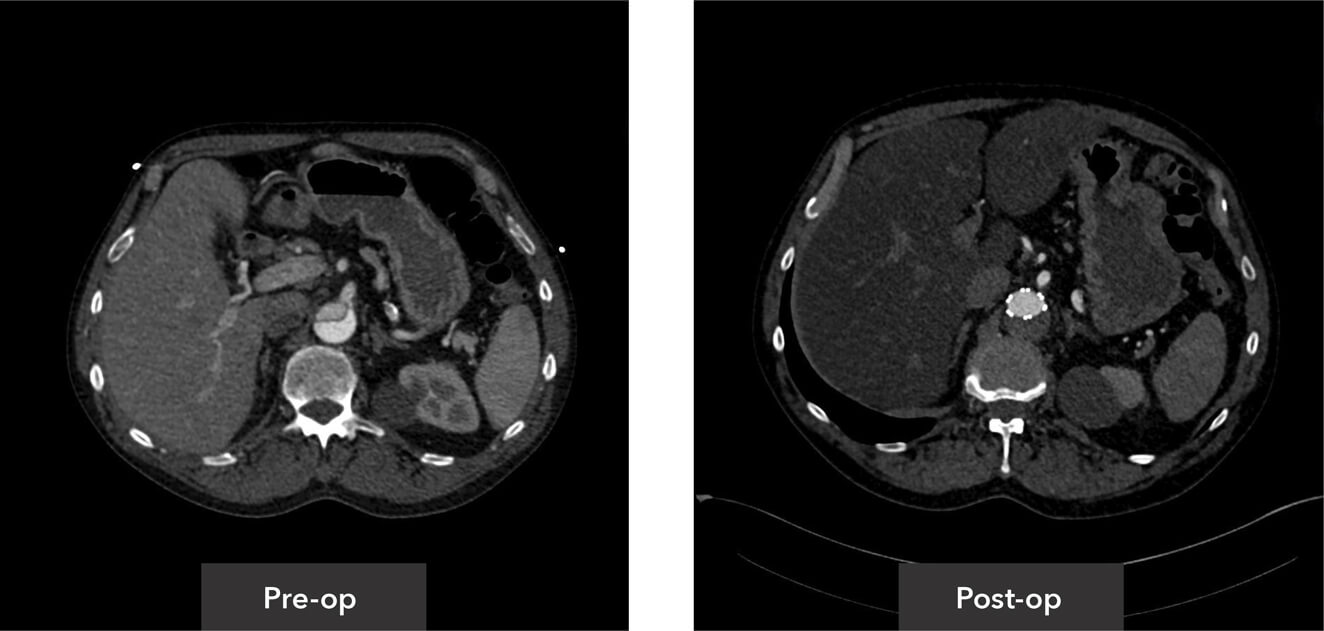
Visceral segment view pre-op/post-op axial view CT imaging
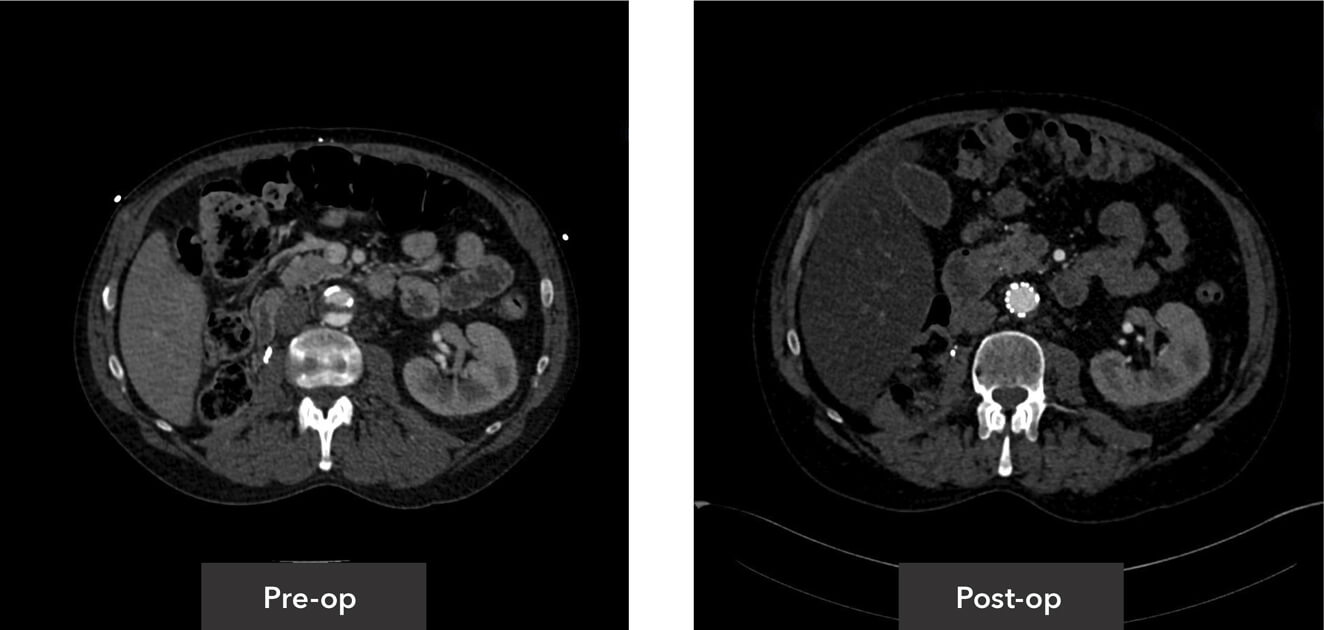
Abdominal aorta view pre-op/post-op axial view CT imaging
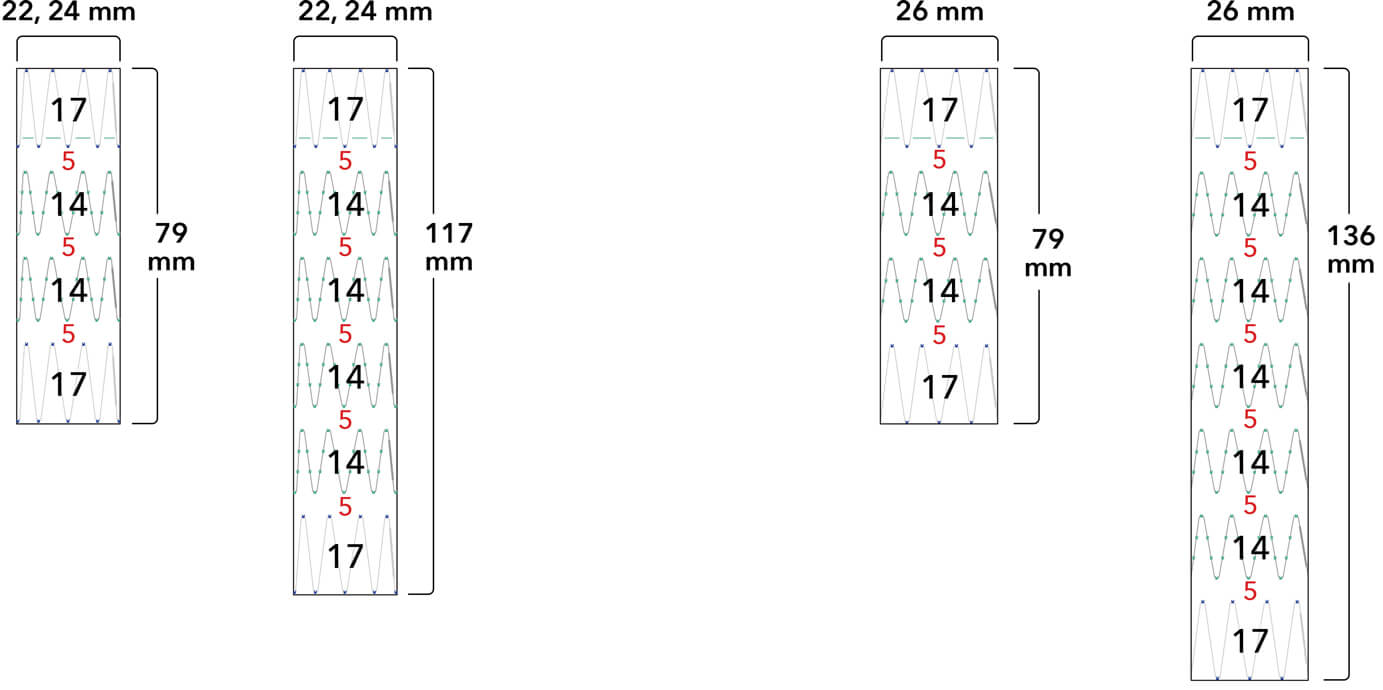
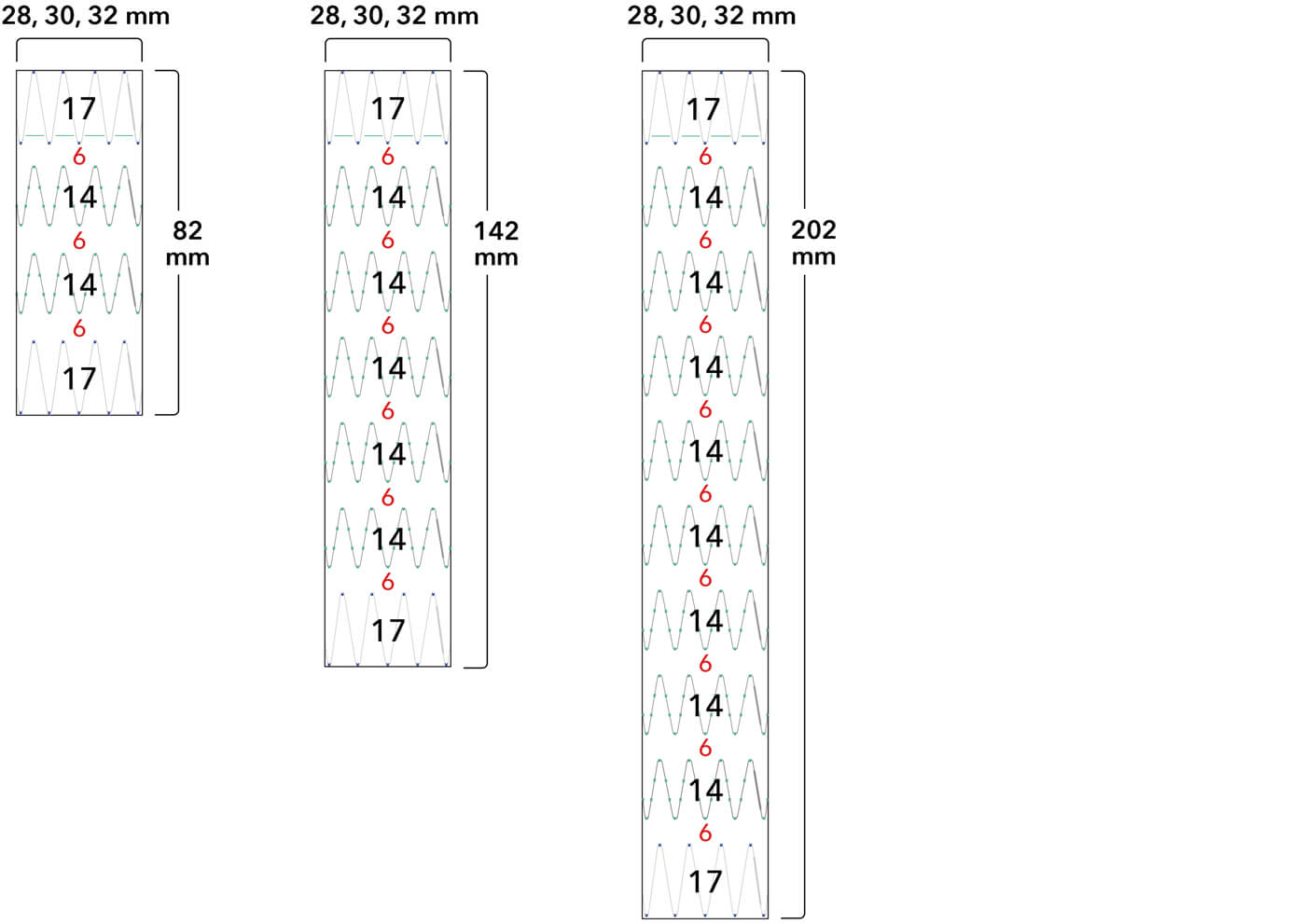
Z-Trak Plus® Introduction system for proximal components
22-34 mm grafts: 20 Fr (6.7 mm) ID/7.7 mm OD
36-42 mm grafts: 22 Fr (7.3 mm) ID/8.5 mm OD
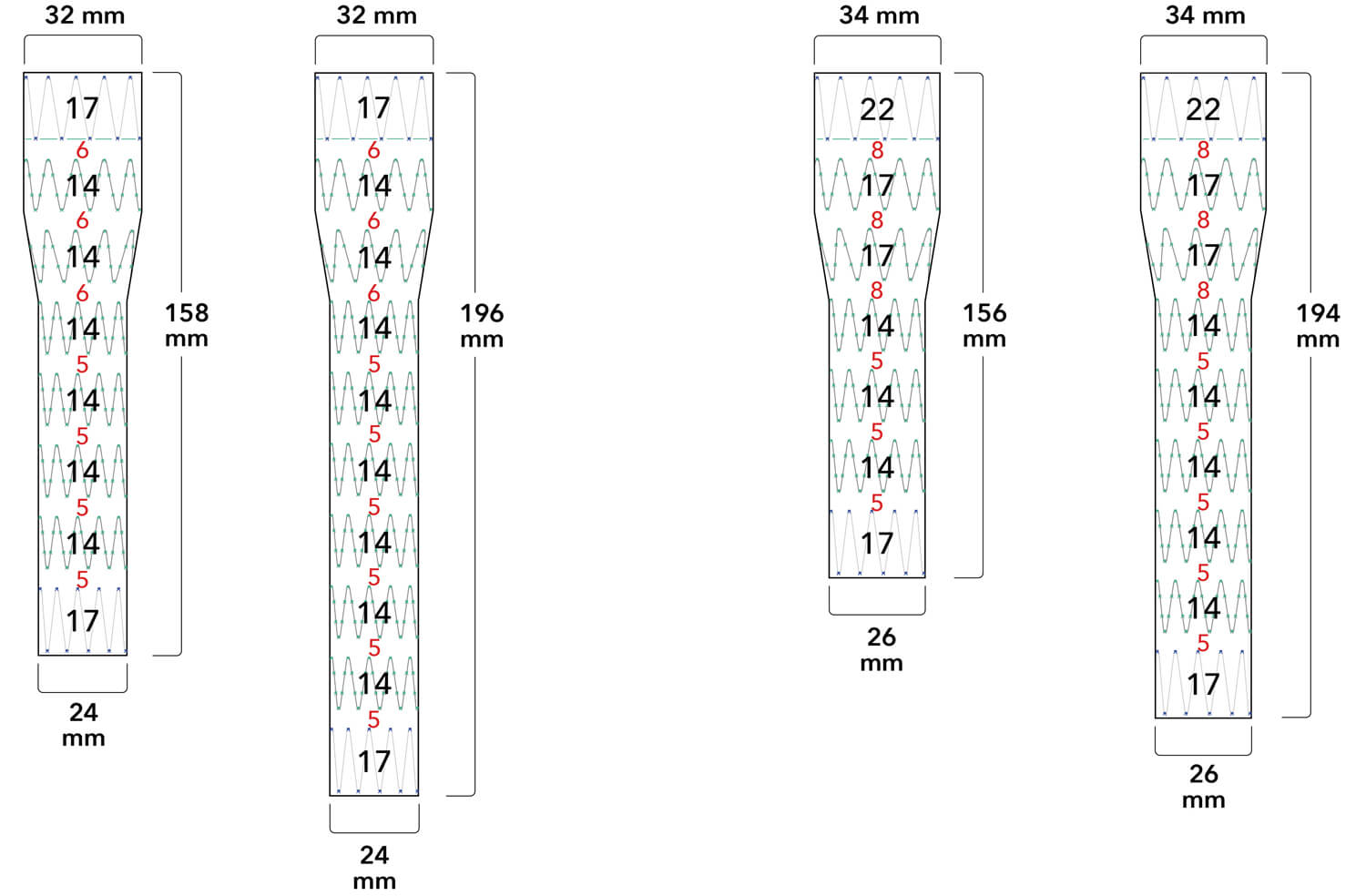
Note: All measurements are in millimeters. Gap lengths are approximate.
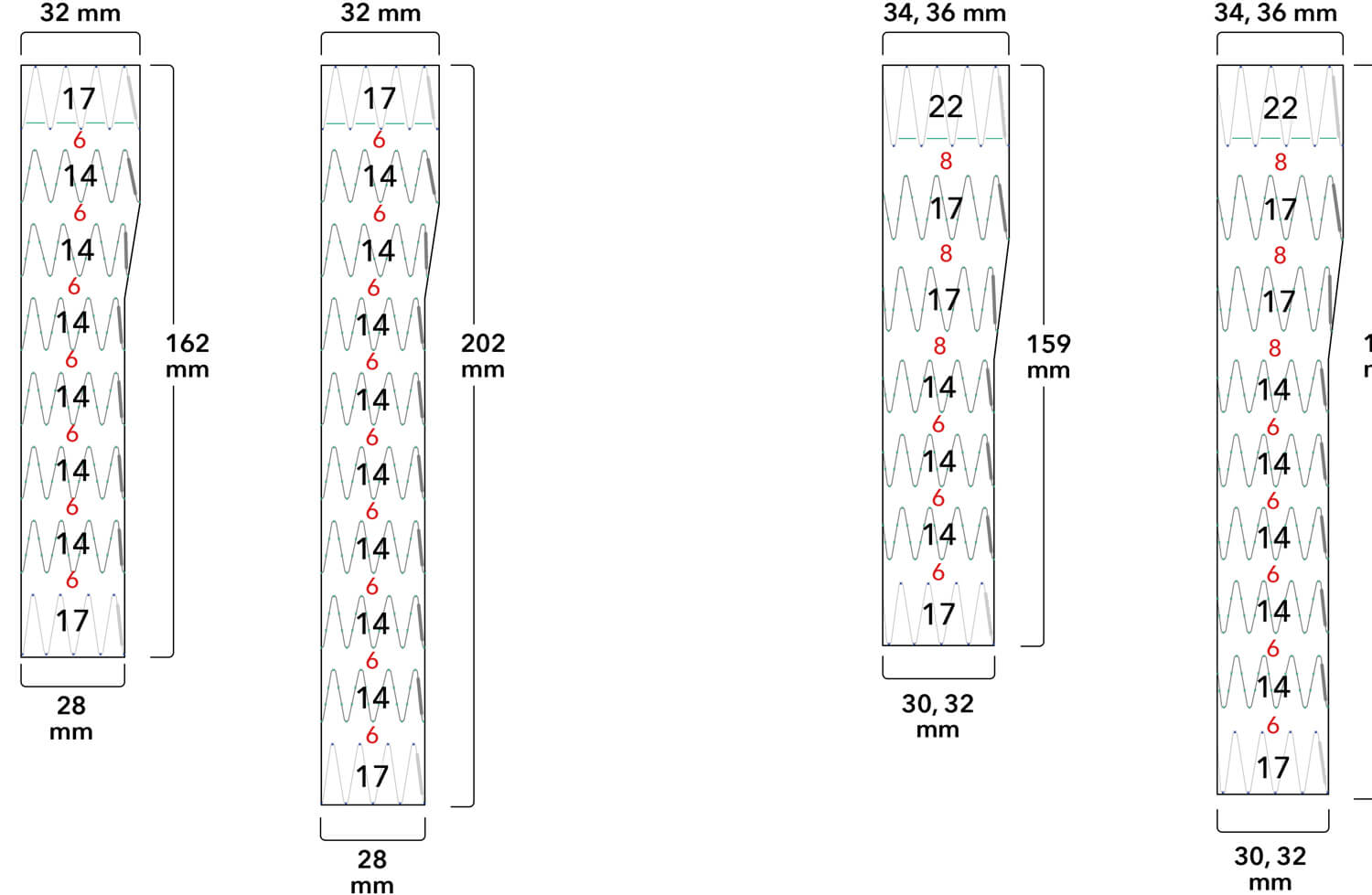
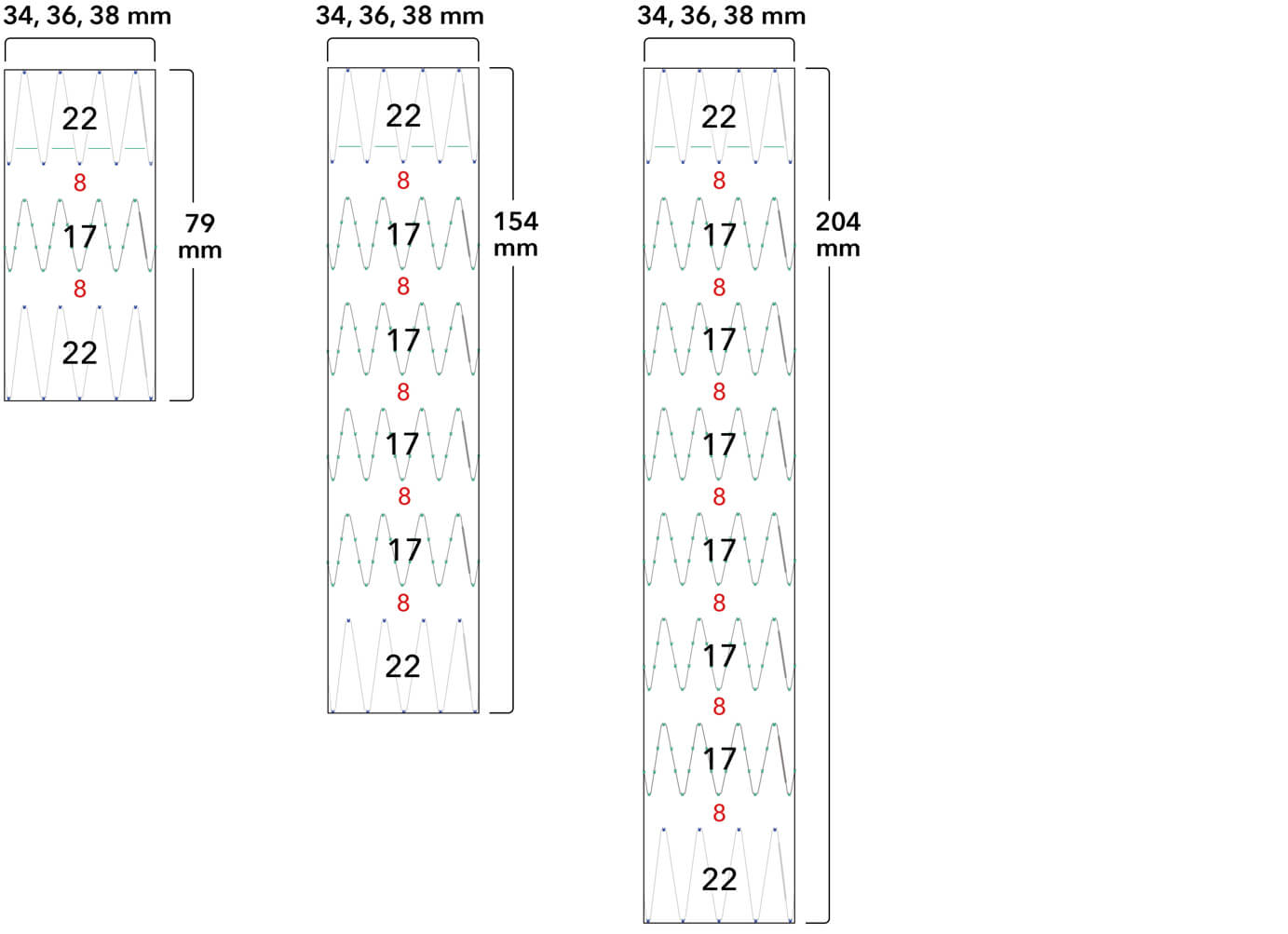
Z-Trak Plus® Introduction system for proximal components
22-34 mm grafts: 20 Fr (6.7 mm) ID/7.7 mm OD
36-42 mm grafts: 22 Fr (7.3 mm) ID/8.5 mm OD

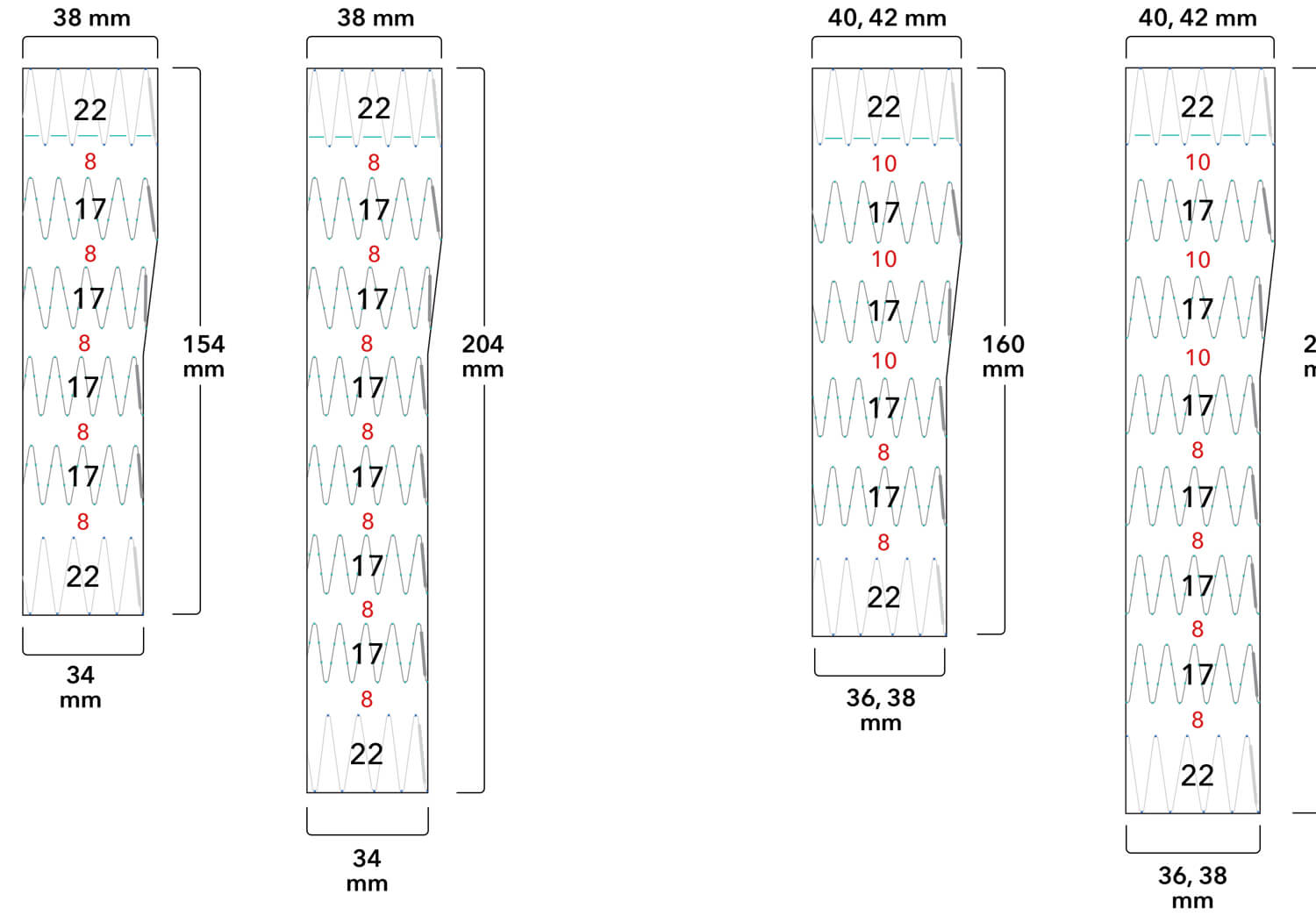
Note: All measurements are in millimeters. Gap lengths are approximate.
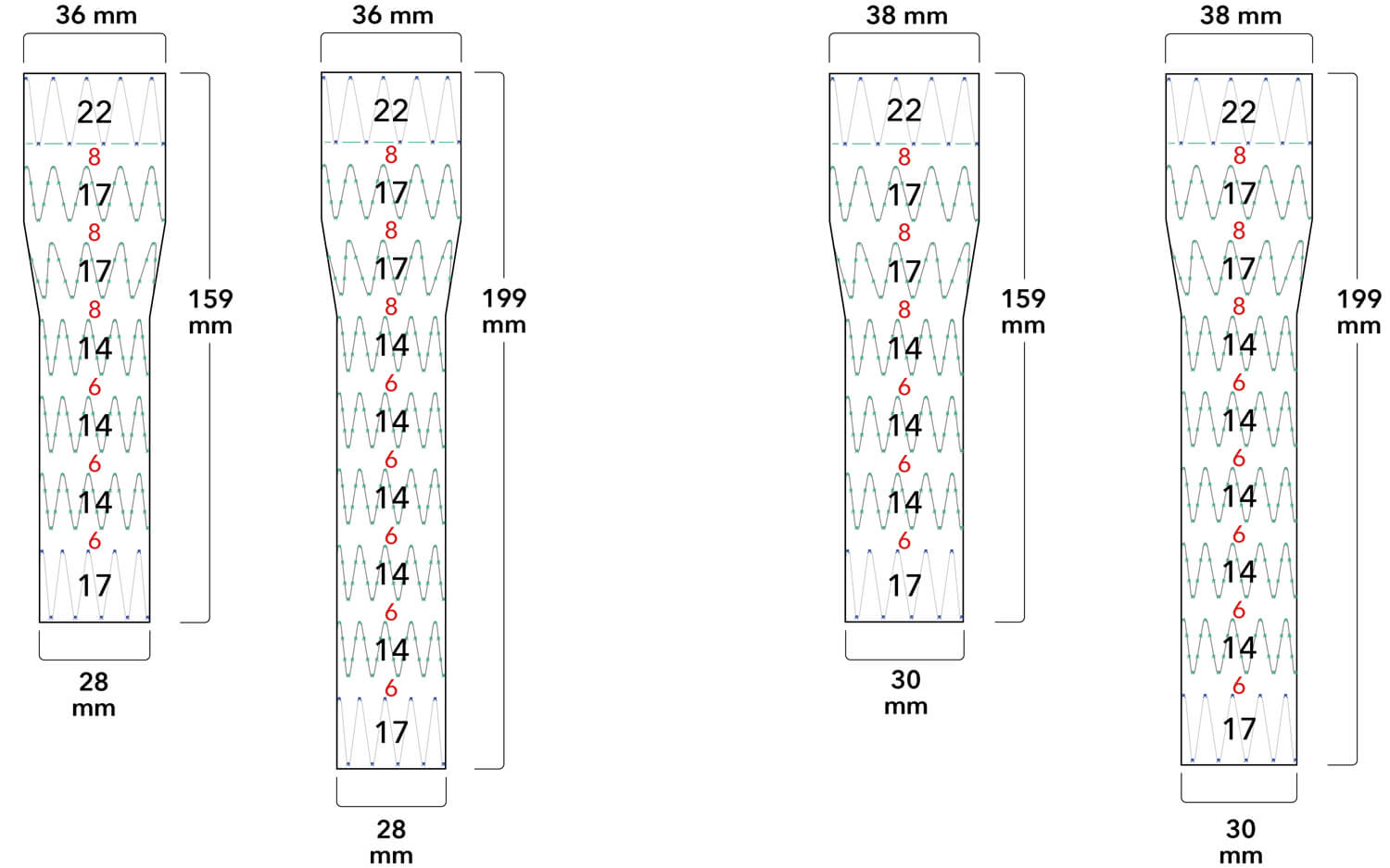
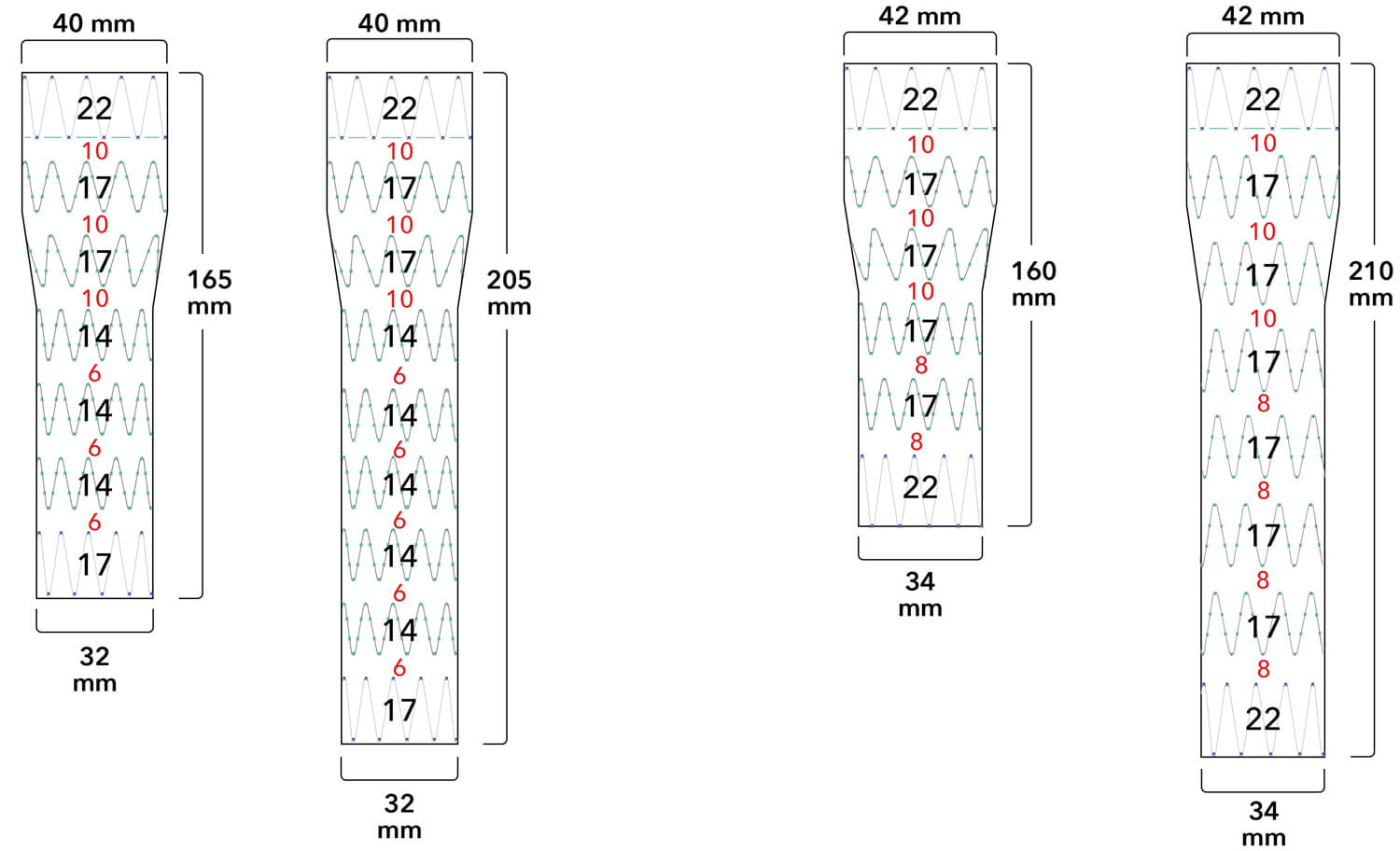
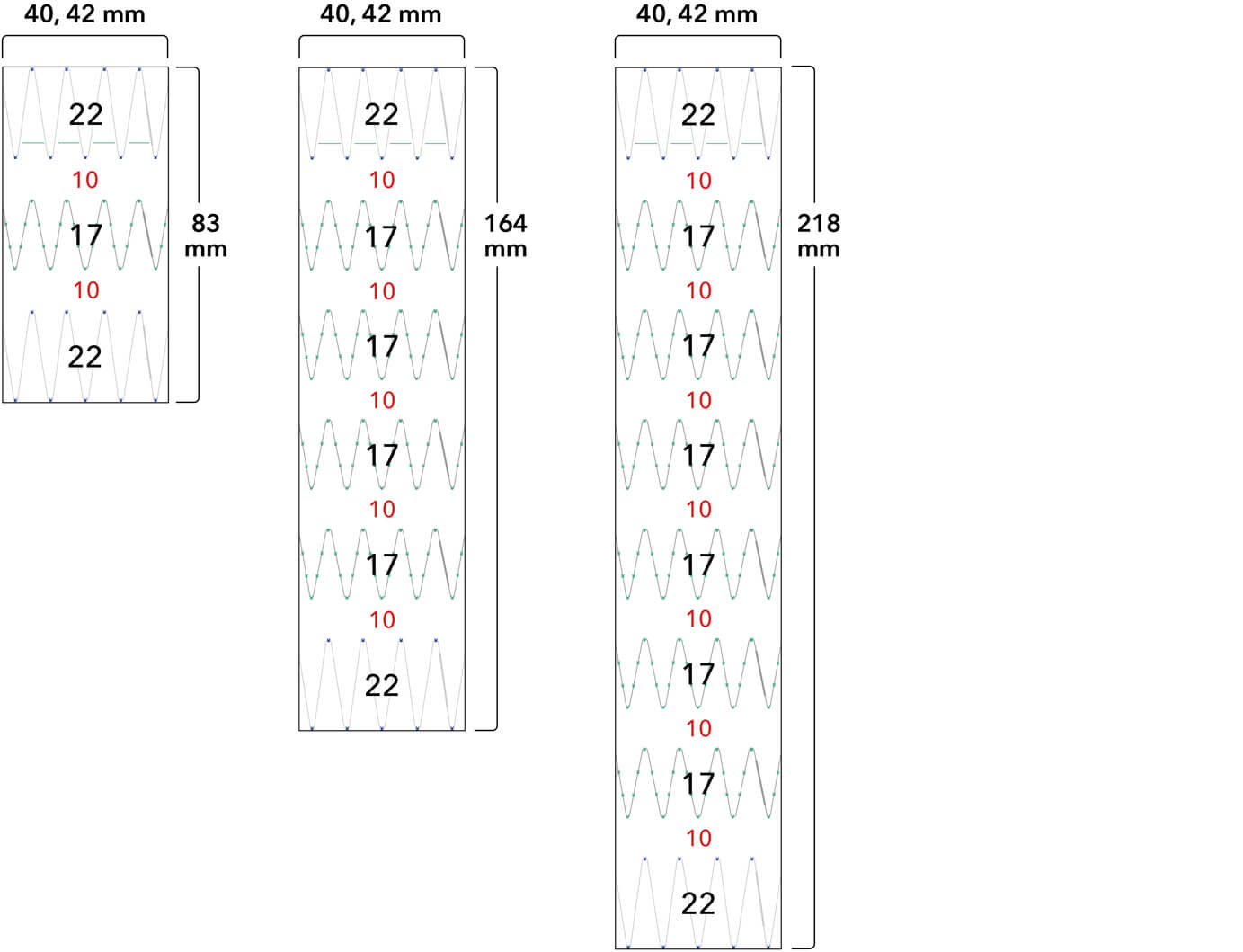
Z-Trak Plus® Introduction system for proximal components
22-34 mm grafts: 20 Fr (6.7 mm) ID/7.7 mm OD
36-42 mm grafts: 22 Fr (7.3 mm) ID/8.5 mm OD

Note: All measurements are in millimeters. Gap lengths are approximate.
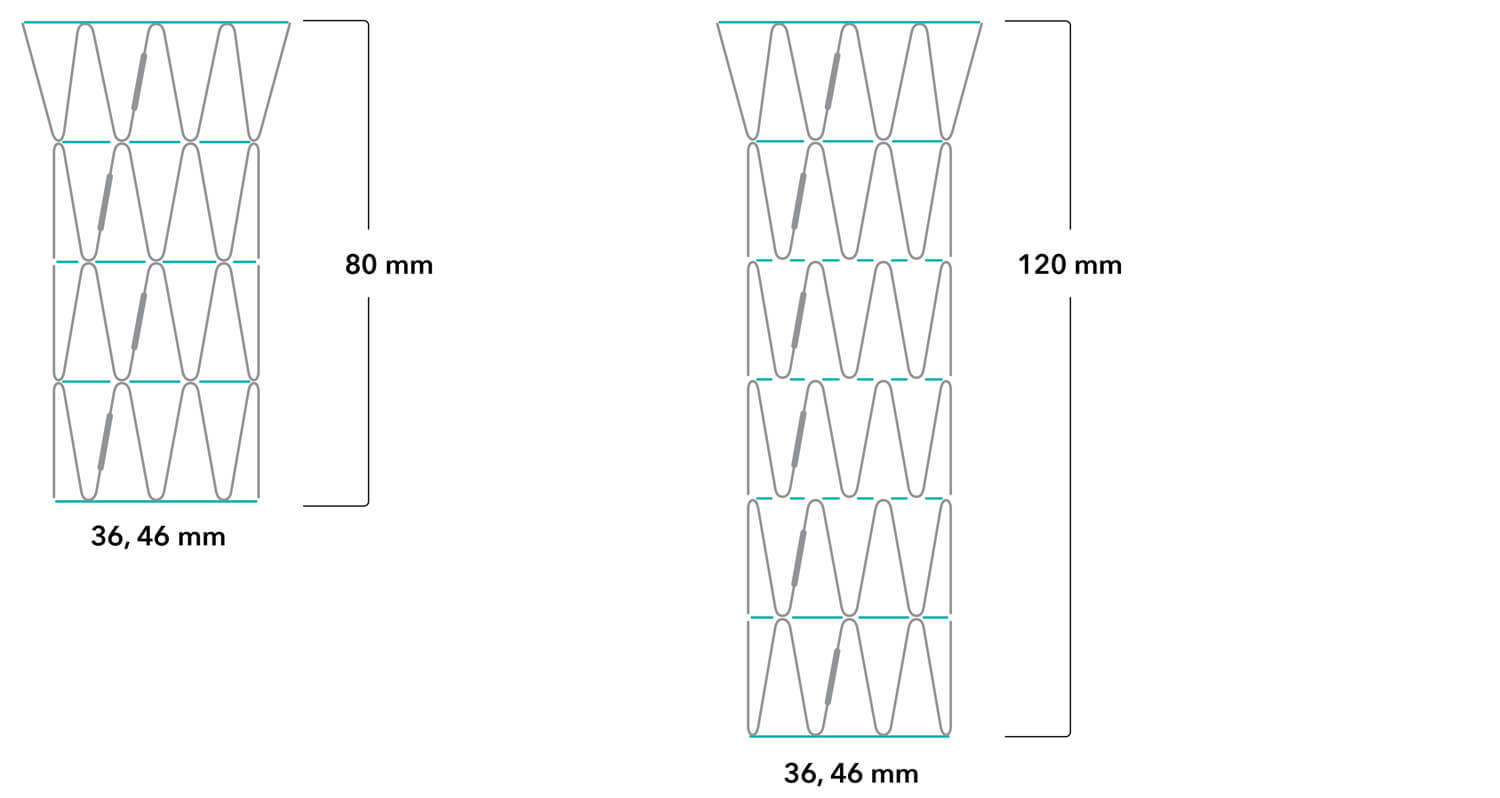
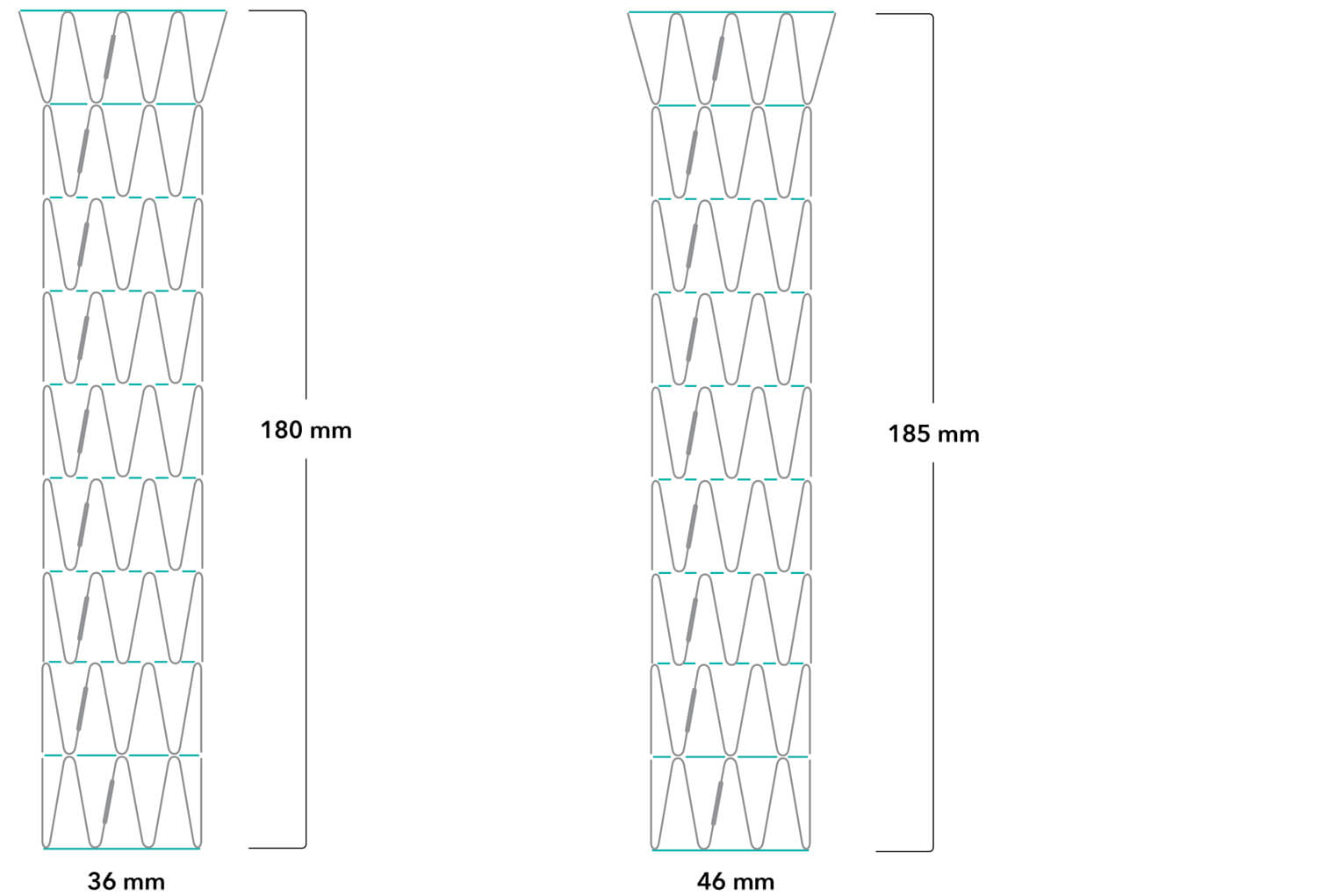
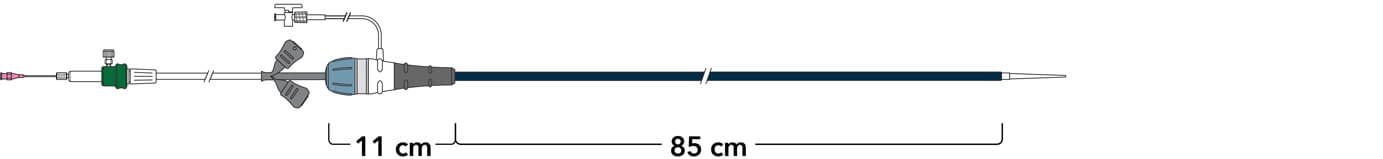
Z-Trak Plus® Introduction system for dissection stents
16 Fr (5.3 mm) ID/6.0 mm OD
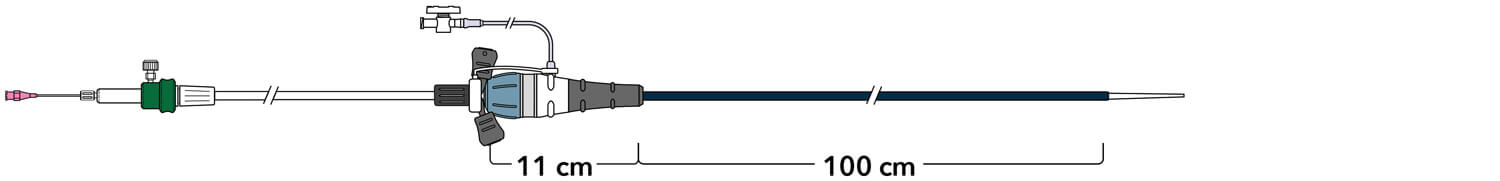
Note: All measurements are in millimeters. Gap lengths are approximate.