The evidence to guide type B aortic dissection decisions and the aims of repair in acute, subacute, and chronic phases, as well as complicated and uncomplicated cases.
BY ATHANASIOS KATSARGYRIS, MD; PABLO MARQUES de MARINO, MD; BALAZS BOTOS, MD; AND ERIC VERHOEVEN, MD, PhD
Over the last decade, type B aortic dissection (TBAD) has gained increasing interest among vascular surgeons, as well as other cardiovascular specialties. Additional scientific knowledge about TBAD was badly needed to address this often complex pathology. Data increasingly demonstrate that TBAD is neither an easy-to-treat nor a benign disease and may have devastating complications in both the acute and chronic phases.
Global registries have shown suboptimal long-term results for medically treated TBAD patients. Dilatation of the false lumen occurs in 20% to 40% of patients over 5 years. Survival rates range from 86% to 100% at 1 year and can be as low as 59% at 5 years. Freedom from aortic events ranges from 34% to 84%.1 The classic definitions of complicated and uncomplicated TBAD have been challenged, and some authors suggest that they should both be considered potential vascular complications requiring repair by an effective and durable strategy.2 Because the disease affects younger patients and many of the deaths during follow-up are aortic-related, the focus is on establishing a treatment that prevents aortic-related complications and mortality in the longer term.
IRAD (International Registry of Aortic Dissection) reported reduced mortality at 5 years in patients with acute TBAD treated by thoracic endovascular aneurysm repair (TEVAR) compared with those who were managed medically.3 Two prospective randomized studies have compared best medical treatment (BMT) alone to BMT + TEVAR for TBAD. The ADSORB trial recruited patients with acute uncomplicated TBAD.4 BMT + TEVAR showed positive aortic remodeling at 1 year compared to BMT alone. The trial, however, was underpowered for mortality at 1-year follow-up.
The INSTEAD trial compared BMT to BMT + TEVAR for patients in stable condition at least 2 weeks after symptom onset (subacute and early chronic phase).2,5 Initial results failed to show a benefit for BMT + TEVAR regarding 2-year cumulative survival rates but showed favorable aortic remodeling in the BMT + TEVAR group. In the later INSTEAD-XL report that analyzed patients during the time interval 2 to 5 years after the index procedure, it was shown that the risk of all-cause mortality (11.1% vs 19.3%; P = .13), aorta-specific mortality (6.9% vs 19.3%; P = .04), and progression of dissection (27.0% vs 46.1%; P = .04) after 5 years was lower for BMT + TEVAR compared to BMT alone. The authors suggested that in patients with stable type B dissection and suitable anatomy, preemptive TEVAR should be considered in order to improve late outcomes.2
INTERVENTIONAL TREATMENT FOR TBAD
TBAD With Acute “Hard” Complications
Rupture, visceral ischemia, and limb ischemia are the feared “hard” complications in acute TBAD. They all require immediate action (“hyperacute” treatment) with damage control aiming for patient survival as a first step. In case of rupture, emergency TEVAR aiming to seal both proximally and distally is the treatment of choice. In case of malperfusion, proximal TEVAR to close the entry tear is the first step. The purpose is to re-expand the true lumen and correct the malperfusion. TEVAR alone may work but, at the same time, may not be enough. It is important to have all available tools on hand to “finish the job.” An important asset is the use of a bare stent as a distal extension over the visceral arteries (Zenith Dissection stent, Cook Medical) to further help the opening of the true lumen by providing support to delaminated segments of the aorta at that level without the risk of covering the visceral arteries. The bare stent may also facilitate additional adjunctive procedures that may be needed in this situation to reestablish organ perfusion (eg, adjunctive visceral or iliac stenting, fenestration techniques that open the dissection flap, open revascularization techniques for visceral arteries or lower limbs). Especially for patients with malperfusion due to dynamic obstruction, endovascular fenestration of the intimal flap can be considered to increase the outflow of the false lumen.6
TBAD With Subacute “Soft” Complications
Although opinions on the topic may vary, refractory pain, uncontrollable hypertension, increasing pleural effusion, rapid aneurysmal expansion, and progressive narrowing of the false lumen are all potential “soft” complications during the initial admission for acute TBAD in our practice. Patients with subacute soft complications are rightfully increasingly considered for TEVAR. Timing for TEVAR in this cohort of patients remains controversial.7,8 In the acute phase, an increased risk of retrograde aortic dissection has been reported.9 Those who recommend waiting at least 2 weeks for the dissection process to settle down justify their choice based on lower perioperative complications with acceptable aortic remodeling rates.2 Those who recommend treating patients in the acute phase believe that the risks are acceptable and aortic remodeling will be maximized the sooner TEVAR is performed.10 In our practice, patients with soft complications are most commonly treated in the subacute phase, usually after a first control CT at day 3 and a second at day 10.
Uncomplicated TBAD With High-Risk Anatomical Features
In our experience, patients without clinical complications but with anatomical features of the dissection associated with a higher risk for future complications can and perhaps should be considered for TEVAR. High-risk anatomical features have been widely studied and include aortic diameter > 4 cm with true and false lumens both patent, rapid expansion of the aortic diameter, primary entry tear diameter ≥ 10 mm, false lumen diameter ≥ 22 mm, large single entry tear in the inner curvature of the aortic arch, etc.11 TEVAR can be considered in this subgroup of patients with the aim to induce positive aortic remodeling and reduce the risk for late complications (ie, aortic dilatation, aneurysm formation, rupture). Timing of TEVAR is again controversial, but a more conservative strategy toward the subacute phase seems logical in our opinion and is also more practical in terms of logistics (ie, time to plan the operation and materials).
In the aforementioned patient categories, TEVAR is considered the first choice above surgery. It is important to realize that TEVAR alone may not “do the job” as previously mentioned. Distal extension with a bare stent (provisional extension to induce complete attachment [PETTICOAT] technique) has been extensively studied in both the STABLE I and II trials in the United States.12 They used the noncovered Zenith Dissection stent and showed a clear benefit with regard to true lumen perfusion. During follow-up, however, no significant reduction of distal aneurysmal degeneration could be demonstrated.
It is important to realize that TEVAR in acute TBAD is not without risks. Devastating complications such as stroke, spinal cord ischemia, and retrograde type A dissection have been reported. Arm ischemia after left subclavian artery coverage is also a serious complication. Stent graft–related complications like collapse, migration, and infection have also been reported during follow-up. The risk of TEVAR-related complications along with the fact that a significant number of TBAD patients will not develop an aneurysm during follow-up means that TEVAR in patients with soft indications sometimes may be an overtreatment, exposing the patient to operative risk without later benefit. A critical appraisal is therefore crucial for selection of suitable patients, despite accumulating data favoring TEVAR.
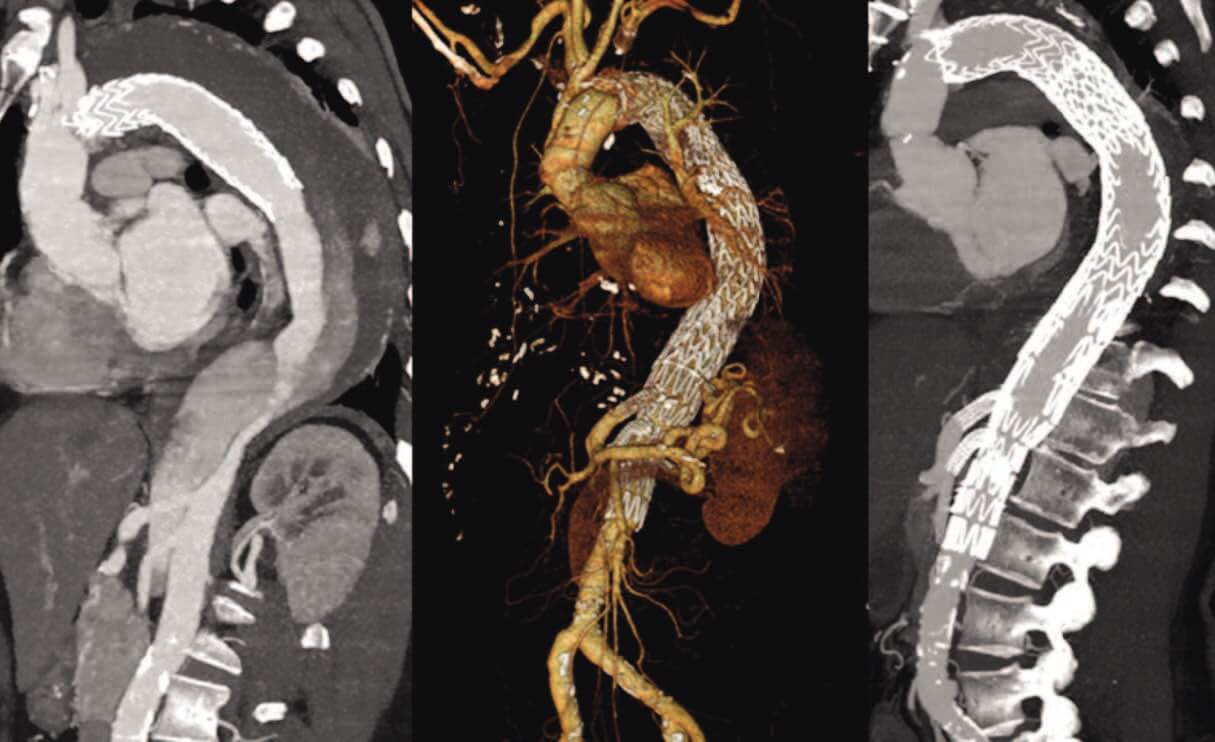
Figure 1. Chronic postdissection TAAA following TEVAR for acute TBAD (A). Treatment with
four-fenestration FEVAR to achieve complete sealing (B, C).
Chronic Postdissection Aneurysms
In chronic TBAD, the indication for treatment is usually the postdissection aneurysm (PDA). The goal of treatment is to exclude the aneurysm to prevent future rupture. This can only be achieved by sealing both proximally and distally. With standard TEVAR, this should only be attempted in those exceptional cases when the PDA is confined to the thoracic descending aorta.13-17 For more extensive thoracoabdominal PDA, a more complex fenestrated and branched endovascular aneurysm repair (F/BEVAR) may be required to exclude all entry and reentry tears, as well as to achieve complete sealing (Figure 1), the availability of which may be restricted/limited depending on the region. For completeness, we report that some authors have used TEVAR plus the Zenith Dissection bare stent to treat selected cases of more extensive PDA. However, the Zenith Dissection bare stent is intended for placement only in nonaneurysmal segments of dissected aorta.
F/BEVAR has been used in recent years to treat PDA of the thoracoabdominal aorta. Additional technical difficulties compared to standard atherosclerotic thoracoabdominal aortic aneurysm (TAAA) include the narrow true lumen, target vessels that originate from the false lumen, and finding/creating adequate proximal and distal sealing zones. Due to these technical difficulties, the experience with F/BEVAR in the treatment of PDA has been limited to a few referral centers.18-21 The first reported experience with F/BEVAR in PDA was published by our group in 2012 and only included six patients.22 In 2014, our combined experience with the University Hospital of Regensburg was published with a total of 31 patients.23 The technical success in this series was 93.5% with a 30-day mortality of 9.6%, reflecting a steep learning curve. Mortality has now regressed below 5% in our personal series of more than 70 patients. The updated published combined experience of Nuremberg and Regensburg includes 71 patients.24 Technical success was 95.8% with an in-hospital mortality of 5.6%. Cumulative survival rates at 12, 24, and 36 months were 84.7% ± 4.5%, 80.7% ± 5.1%, and 70.0% ± 6.7%, respectively. Mean aneurysm sac regression during follow-up was 9.2 ± 8.8 mm, with a false lumen thrombosis rate of 85.4% for patients with a follow-up longer than 12 months. No ruptures occurred during follow-up, showing that F/BEVAR can be a safe and effective treatment for extensive thoracoabdominal PDA.
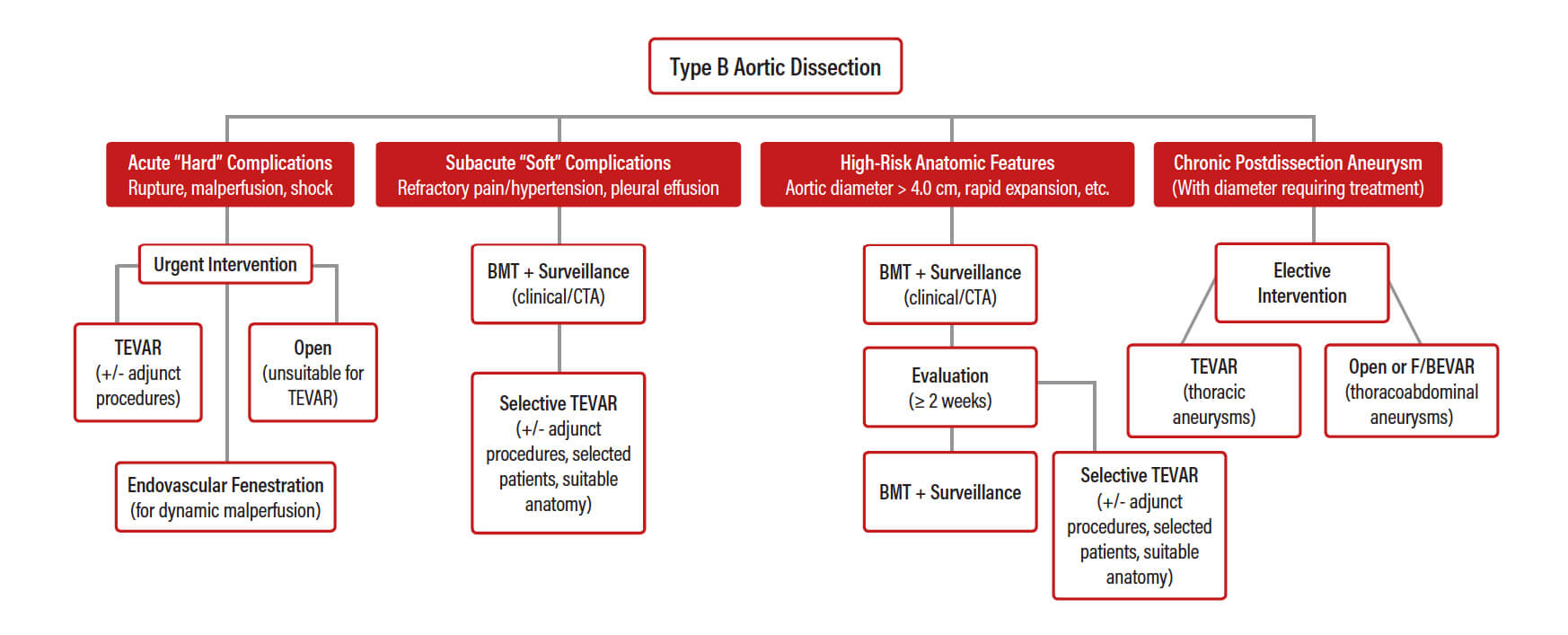
Figure 2. Authors’ proposed treatment algorithm for TBAD.
Personal Treatment Algorithm for TBAD Patients
Where do we stand today in terms of decision-making for TBAD? According to the available evidence, urgent TEVAR should be the first-line intervention in patients with acute (hard) complicated TBAD. For patients with subacute (soft) complications and/or anatomic features that predispose them to future complications, TEVAR should probably be considered on an individual basis in the subacute phase. Finally, for chronic PDA, standard TEVAR has only a limited role in patients where distal sealing can be achieved in the thoracic aorta. For more extensive PDA, adequate sealing requires the use of F/BEVAR. This is summarized in a treatment algorithm proposed by the authors (Figure 2).
REMAINING QUESTIONS AND FUTURE PERSPECTIVES
Despite evident progress in the understanding and management of TBAD during the last decade, several questions remain unanswered. Further studies aiming to define subgroups of patients who are more likely to have late aortic events and therefore justify early treatment with TEVAR are needed. More evidence is also needed with regard to the best timing for TEVAR, especially for patients who can wait without missing the best treatment window to maximize aortic remodeling. Late distal aneurysmal degeneration after both medical treatment and TEVAR for acute TBAD is a serious concern and has led to the evolution of several adjunctive endovascular techniques to counteract distal aortic dilatation.
The PETTICOAT technique with additional stenting over the visceral arteries using the Zenith Dissection stent has demonstrated benefits with regard to true lumen diameter but failed to show a clear advantage with regard to prevention of aneurysmal dilatation. In Europe, a new adjunctive technique is being evaluated by physicians (without industry involvement or support due to its off-label use), the stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair (STABILISE) concept, which includes the use of a stent graft to cover the proximal entry tear (TEVAR), followed by a noncovered stent over the visceral arteries (like PETTICOAT), and then additional ballooning with a larger balloon to disrupt the dissection flap with the aim of obliterating the false lumen and restoring single-lumen flow.25-27 In theory, the technique seems to be a serious attempt to “cure” dissection patients and prevent late aneurysmal degeneration, but more studies are required before widespread use can be advocated. The European registry on the STABILISE concept was created by Melissano and colleagues25 and aims to collect data from multiple European centers to monitor the technique in the long-term with the hope of providing some answers to these remaining questions.
Athanasios Katsargyris, MD
General Hospital Nuremberg
Paracelsus Medical University
Nuremberg, Germany
Disclosures: None.
Pablo Marques de Marino, MD
General Hospital Nuremberg
Paracelsus Medical University
Nuremberg, Germany
Disclosures: None.
Balazs Botos, MD
General Hospital Nuremberg
Paracelsus Medical University
Nuremberg, Germany
Disclosures: None.
Eric Verhoeven, MD, PhD
Chief, Department of Vascular and Endovascular Surgery
General Hospital Nuremberg
Paracelsus Medical University
Nuremberg, Germany
eric.verhoeven@klinikum-nuernberg.de
Disclosures: Speaker, proctor, consultant, and research grants from Cook Medical.
- Moulakakis KG, Mylonas SN, Dalainas I, et al. Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg. 2014;3:234-246.
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407-416.
- Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6:876-882.
- Brunkwall J, Kasprzak P, Verhoeven E, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48:285-291.
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the investigation of stent grafts in aortic dissection (INSTEAD) trial. Circulation. 2009;120:2519-2528.
- Riambau V, Böckler D, Brunkwall J, et al. Editor’s choice 2013—Management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4-52.
- Song C, Lu Q, Zhou J, et al. The new indication of TEVAR for uncomplicated type B aortic dissection. Medicine. 2016;95:e3919.
- Conrad MF, Crawford RS, Kwolek CJ, et al. Aortic remodeling after endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2009;50:510-517.
- Chen Y, Zhang S, Liu L, et al. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e004649.
- Qin YL, Wang F, Li TX, et al. Endovascular repair compared with medical management of patients with uncomplicated type B acute aortic dissection. J Am Coll Cardiol. 2016;67:2835-2842.
- Acosta S, Blomstrand D, Gottsater A. Epidemiology and long-term prognostic factors in acute type B aortic dissection. Ann Vasc Surg. 2007;21:415-422.
- Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629-640.e2.
- Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg. 2011;142:1074-1083.
- Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg. 2012;43:386-391.
- Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg. 2008;36:522-529.
- Manning BJ, Dias N, Ohrlander T, et al. Endovascular treatment for chronic type B dissection: limitations of short stent-grafts revealed at midterm follow-up. J Endovasc Ther. 2009;16:590-597.
- Scali ST, Feezor RJ, Chang CK, et al. Efficacy of thoracic endovascular stent repair for chronic type B aortic dissection with aneurysmal degeneration. J Vasc Surg. 2013;58:10-17.e1.
- Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg. 2013;58:625-634.
- Spear R, Sobocinski J, Settembre N, et al. Early experience of endovascular repair of post-dissection aneurysms involving the thoraco-abdominal aorta and the arch. Eur J Vasc Endovasc Surg. 2016;51:488-497.
- Spear R, Hertault A, Van Calster K, et al. Complex endovascular repair of postdissection arch and thoracoabdominal aneurysms. J Vasc Surg. 2018;67:685-693.
- Law Y, Tsilimparis N, Rohlffs F, et al. Fenestrated or branched endovascular aortic repair for postdissection thoracoabdominal aortic aneurysm. J Vasc Surg. 2019;70:404-412.
- Verhoeven EL, Paraskevas KI, Oikonomou K, et al. Fenestrated and branched stent-grafts to treat post-dissection chronic aortic aneurysms after initial treatment in the acute setting. J Endovasc Ther. 2012;19:343-349.
- Oikonomou K, Kopp R, Katsargyris A, et al. Outcomes of fenestrated/branched endografting in post-dissection thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;48:641-648.
- Oikonomou K, Kasprzak P, Katsargyris A, et al. Mid-term results of fenestrated/branched stent grafting to treat post-dissection thoraco-abdominal aneurysms. Eur J Vasc Endovasc Surg. 2019;57:102-109.
- Melissano G, Bertoglio L, Rinaldi E, et al. Satisfactory short-term outcomes of the STABILISE technique for type B aortic dissection. J Vasc Surg. 2018;68:966-975.
- Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg. 2014;147:1240-1245.
- Faure EM, El Batti S, Abou Rjeili M, et al. Mid-term outcomes of stent assisted balloon induced intimal disruption and relamination in aortic dissection repair (STABILISE) in acute type B aortic dissection. Eur J Vasc Endovasc Surg. 2018;56:209-215.
