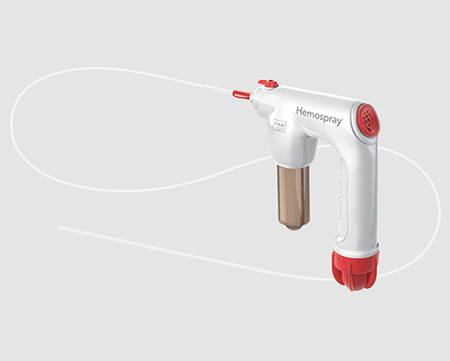
 Hemospray Endoscopic Hemostat has returned to the market and is available to order.
Hemospray Endoscopic Hemostat has returned to the market and is available to order.
In January, Cook Medical initiated a voluntary global recall of Hemospray Endoscopic Hemostat after receiving complaints that the handle and/or activation knob had cracked or broken when the device was activated. The recall applied to all lots manufactured from January 16, 2017, to January 15, 2020.
Cook Medical has implemented a new production test to address concerns with potential cracking or breaking of the activation knob. Now 100% of the activation knob components are inspected to ensure that they exceed worst-case forces applied to the activation knob during activation of the device. No changes have been made to the Hemospray Endoscopic Hemostat device’s delivery system or functionality.
For more information on Hemospray, please visit hemospray.cookmedical.com.
