Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients.
We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more ways Cook is changing to better serve our customers.
1. Growing our product portfolio to fit our future
We’ve recently adjusted our product portfolio to better offer products that align with our vision and serve unmet customer needs with a unique portfolio of products. These portfolio changes include:
Investing in Zenflow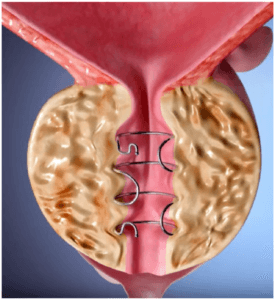
Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment for urinary obstruction caused by enlarged prostate, or benign prostatic hyperplasia (BPH).“This investment reflects our confidence in the future of Zenflow and the Spring® System technology. Zenflow is aligned with our core focus of developing minimally invasive technologies that restore flow,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division. “This type of agreement is yet another proof-point to how Cook is changing to focus on our future as product innovators.”
Investing in PillSense
Cook Medical signed an agreement to distribute EnteraSense’s novel blood-sensing capsule, PillSense. We also invested in EnteraSense’s Series B round of funding to support the production and distribution of PillSense capsules. PillSense is a strategic addition to Cook’s portfolio of endoscopic bleed management products, such as Hemospray and the Instinct Plus™ Endoscopic Clipping Device.
Divesting Lead Management products
Cook Medical sold our portfolio of lead management products to Merit Medical. Merit has an existing footprint in the electrophysiology (EP) space and plans to expand their presence in Lead Management. Merit is making significant investment in EP and shows they are committed to growing the business in ways that Cook has not been able to in recent years. This acquisition represents a tremendous opportunity for employees, products and patients while aligning with Cook’s long-term goals.
Divesting the Reproductive Health portfolio
Cook Medical sold our portfolio of Reproductive Health and Assisted Reproductive Technology (ART) products to Astorg. Astorg, an investment firm with deep experience in MedTech across both manufacturing and product businesses with a focus on growth and innovation, acquired and simultaneously combined the Cook ART portfolio with Hamilton Thorne, a leading provider of precision instruments, consumables, software, and services to the ART research and the cell biology space.
“Part of achieving our business strategy has included reviewing our product portfolio and identifying what product lines are the best fit for Cook moving forward and which might have more opportunity to grow or thrive elsewhere,” said DJ Sirota, senior vice president of Cook Medical’s MedSurg division.
“This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties to align with Cook’s more focused vision for the future of the company,” said Pete Yonkman, president of Cook Medical and Cook Group.
2. Innovating our training with new product simulators
 If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
If you’ve been to VIVA or VEITH conferences recently, you might have noticed extra attention at the Cook booths. It’s because we brought our new product simulators!
Product simulators are an important element of Cook’s comprehensive medical education program. These simulators allow residents, fellows and physicians of all experience levels to get a feel—literally—for how our products work. In addition to providing important practice for treating serious disease states, simulators are good for honing technique and getting used to how products work so physicians are prepared to treat patients to the best of their ability.
In addition to the PAD simulators we’ve had for several years, we’ve recently made new product simulators for the Zenith Fenestrated AAA Endovascular Graft and for embolization coils. You can see them at large industry conferences, as well as certain Cook Vista medical education courses. You can sign up to reserve them for a training session.
3. Building World-First Device Testing Machines
Cook is testing the limits of what’s possible with our devices. Currently, there is no commercially available equipment that can test the longevity of certain materials (like superelastic nitinol, often used in vascular medical devices) in certain loading conditions (like heart beats). To create products that are stronger and last longer, we decided to create the world’s first machine to test these materials that experience hundreds of millions of heart-beat loads.

We partnered with Dynatek Labs, a world leader in medical device testing, equipment,services and consulting. Together we created the first fatigue-to-fracture equipment that tests superelastic alloys in cardiac pulsatile loading. This machine simulates the wear and tear a typical nitinol peripheral stent experiences over time, including electrical pulses and physical movement. The machine simulates the conditions until the device starts to fracture. With this data, we can accurately predict how long materials will last and make safer and more durable devices.
The research on the machine was published in ASTM International and you can read the details here.
4. Introducing Cook Leadership
Get to know the leaders at Cook Medical! Our new Meet Our Leaders series introduces key executives at Cook. You’ll get to hear about their background, what they love about Cook and why they’re passionate about what they do. Here are some of the Cook leaders you can get to know:
- Dr. John Kaufman, chief medical officer
- DJ Sirota, senior vice president, MedSurg division
- Christa Curtis, vice president of global marketing and communications
- Ross Harvey, vice president of global supply chain
Be sure to follow Cook Medical on social media and frequently check our newsroom to learn more about Cook’s leaders and how their expertise is shaping the future of medical devices.