Dear colleagues,
Over the past few months, there has been a lot of discussion regarding the use of paclitaxel for the treatment of peripheral arterial disease. We want to share with you our perspective and some important information on this topic.
Patient safety is our first priority.
We are committed to gaining a better understanding of the recent information regarding paclitaxel-eluting devices. We are sharing data we’ve gained over the past 20 years researching paclitaxel and developing Zilver PTX, including information on safety and effectiveness. Cook’s paclitaxel-coated stent was approved by the FDA in 2012 through the premarket approval process.
FDA has provided guidance to healthcare providers.
On March 15, the FDA issued an updated letter that outlines current recommendations to healthcare providers who are making treatment decisions about paclitaxel devices. We recommend that you follow the FDA guidelines. A full version of the letter is available at www.fda.gov, and includes the following recommendations.
Based on the FDA’s preliminary review of available data, we recommend that health care providers consider the following until further information is available:
- Continue diligent monitoring of patients who have been treated with paclitaxel-coated balloons and paclitaxel-eluting stents.
- When making treatment recommendations and as part of the informed consent process, consider that there may be an increased rate of long-term mortality in patients treated with paclitaxel-coated balloons and paclitaxel-eluting stents.
- Discuss the risks and benefits of all available PAD treatment options with your patients. For most patients, alternative treatment options to paclitaxel-coated balloons and paclitaxel-eluting stents should generally be used until additional analysis of the safety signal has been performed.
- For some individual patients at particularly high risk for restenosis, clinicians may determine that the benefits of using a paclitaxel-coated product may outweigh the risks.
- Ensure patients receive optimal medical therapy for PAD and other cardiovascular risk factors as well as guidance on healthy lifestyles including weight control, smoking cessation, and exercise.
Additionally, the FDA noted that the data used to develop the recommendations should be interpreted with caution for several reasons:
- First, there is large variability in the risk estimate of mortality due to the limited amount of long-term data.
- Second, these studies were not originally designed to be pooled, introducing greater uncertainty in the results.
- Third, the specific cause and mechanism of the increased mortality is unknown.
Cook is collaborating with FDA and other global regulatory agencies.
We are working to provide answers to questions from the FDA and other global regulatory authorities, including providing patient level data. The FDA has also asked industry to collaborate and present at an FDA panel meeting in June. Cook is helping lead the industry-wide working group that is preparing data for presentation at this panel meeting. We are also collaborating with medical societies and have been sharing our data to provide a deeper understanding of our mortality analysis.
Information about Zilver PTX data.
There has been a lot of discussion in recent weeks and we realize that you are striving to make the best decisions for your patients. We want to provide you with facts and information about Zilver PTX, our clinical trial, and the resulting data.
Trial Design:The Zilver PTX RCT had a unique trial design. The RCT included a primary and secondary randomization, as well as an opportunity for patients in the PTA arm to cross over to treatment with Zilver PTX after experiencing a target lesion revascularization (TLR) in the first 12 months. As a result of this trial design, 40% of patients who were initially assigned to the PTA arm were subsequently treated with Zilver PTX. In total, 70% of the patients enrolled in this trial received a Zilver PTX stent.
“Intent to treat” versus “As treated”:To appropriately analyze the role of paclitaxel on mortality, it is important to compare all patients who were treated with Zilver PTX to those treated only with non-drug-eluting devices.
The “intent to treat” analysis shows a 5-year all cause mortality rate of 22.1% for the Zilver PTX primary randomization arm and 15.3% for the PTA primary randomization arm (p-value = 0.04). However, this analysis does not recognize that 94 of the 237 patients (40%) in the primary PTA arm were actually treated with a Zilver PTX stent yet analyzed as PTA patients and not Zilver PTX patients in this intent to treat analysis.
The “as treated” analysis shows a mortality rate of 18.7% for Zilver PTX patients and 17.6% for PTA/BMS patients (p-value = 0.53). This “as treated” Zilver PTX group includes all patients who received a Zilver PTX stent.
Cause of death: Causes of death were examined between the “as-treated” Zilver PTX and PTA/BMS groups. There was no increased rate of cardiovascular, cancer, or other cause of death for Zilver PTX compared to PTA/BMS.
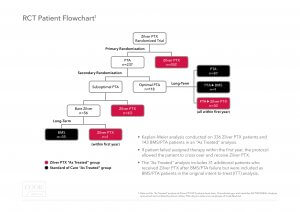
Results from our “as treated” analysis show no statistical difference in mortality for the Zilver PTX group and for the PTA/BMS group.
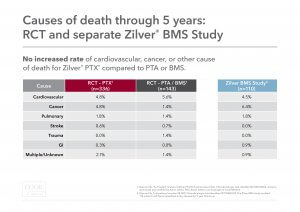
This chart shows the 5-year cause of death.
Cook is committed to serving patients and clinicians.
During this time, we are committed to supporting our customers and to responding to the questions and concerns of medical societies and regulatory agencies. We will continue to share what we’ve discovered from our research and data in the area of paclitaxel.
If you have questions about Zilver PTX, please contact us at zilverptx@cookmedical.com, and a member of our team will respond within 24 hours. You can also visit cookmedical.com for additional information.
Sincerely,
Aaron Lottes, PhD
Director, Regulatory Science
Cook Research, Inc.
Mark Breedlove
Vice President, Vascular Division
Cook Medical
Bloomington, Ind. — Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the descending thoracic aorta. The device will be available for sale in the U.S. in the coming months.
“We’re pleased to provide another minimally invasive option for aortic repair,” said Mark Breedlove, vice president of Cook Medical’s Vascular division. “The approval of this product gives us an opportunity to have a positive impact on the lives of patients with aortic dissections.”
Aortic dissection is a tear that occurs between the innermost and middle layers of the aorta. When the inner layer of the aorta tears, blood flows through the tear, which causes the inner and middle layers of the aorta to separate (dissect). Type B dissection involves a tear in the lower (descending) aorta, while Type A dissection involves a tear in the upper (ascending) aorta.
Globally, thoracic endovascular aortic repair (TEVAR) is acknowledged as the treatment of choice for complicated Type B aortic dissection. These procedures are meant to prevent malperfusion of aortic branches and aortic rupture.
“Cook Medical is committed to developing a variety of treatment options for aortic disease – from the arch to the iliacs, in order to help physicians fit a device to each patient’s unique disease state,” Breedlove said.
To learn more about Cook’s disease-specific treatment options for endovascular repair, visit aortic.cookmedical.com.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Winston-Salem, N.C. – Today, Cook Medical announced that it has completed an agreement with Whitaker Park Development Authority Inc. to acquire a long-standing facility at Winston-Salem’s Whitaker Park. The agreement was completed on December 27, 2018. Cook will begin converting a portion of the R.J. Reynolds building in Whitaker Park into a world-class medical device manufacturing facility this year.
“Cook has a history of turning unused properties into valuable assets in our communities,” said Barry Slowey, president of Cook Medical’s Winston-Salem location. “We see Whitaker Park as an impact project in that we are converting a facility that manufactured cigarettes into a modern facility producing life-saving medical devices. We’ve been in Winston-Salem since 1983, and acquiring the Whitaker Park facility signifies a renewed commitment to this community.”
Built in 1961, the 850,000-square-foot facility will host Cook Medical’s current workforce of more than 650 employees in Winston-Salem. Cook anticipates adding 50 new jobs over the next 10 years.
“This space will offer us additional flexibility with production capabilities and represents our continued investment in the local community,” Slowey continued. “Whitaker Park presents tremendous opportunities for us to continue developing and manufacturing medical devices for patients around the world.”
About Cook Medical
Since 1963 Cook Medical has worked closely with clinicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.

Cook Global Headquarters, Bloomington, Indiana.
Bloomington, Ind. — Last week, Cook Medical was recognized as one of the best places to work in Bloomington. The honor comes from the Bloomington Economic Development Corporation in partnership with The Mill and The Herald-Times.
“We’re pleased to be included in the inaugural class for the Bloomington Best Places to Work. This award is special because it recognizes the importance of company culture and its connection to a successful business.” said Pete Yonkman, president of Cook Group and Cook Medical. “As a family company, we have been able to create a unique culture that provides meaningful work that we all feel good about at the end of the day.”
An important part of the company’s culture is an open environment that encourages collaboration and feedback. Cook employees have continual opportunities to provide feedback to company leadership. Many of those suggestions have been implemented, leading to changes like creating the employee health clinic, process improvements, new benefits like parental leave, and updates to the company’s education and professional development program, My Cook Pathway.
“Programs like My Cook Pathway have allowed us provide employees with opportunities to further their education,” said Nicky James, vice president of Human Resources and Talent Development for Cook Group and Cook Medical. “From helping employees earn their high school diplomas to obtaining professional development opportunities, we want to empower all employees to reach their personal and professional goals.”
Cook Medical has more than 10,000 employees around the world, 70 percent of whom are located in the U.S.
The Bloomington Best Places to Work recognition for Cook Medical follows Forbes naming the company as one of America’s Best Employers of the Year in 2018.
To learn more about careers at Cook Medical, visit cookmedical.com/careers.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.

Hemospray®
Bloomington, Ind. – The FDA has granted Cook Medical approval to market Hemospray®, in the United States. An endoscopic hemostat, Hemospray achieves hemostasis with a proprietary inorganic powder. Bleeding in the gastrointestinal tract is a leading cause of morbidity and is associated with an estimated 20,000 deaths per year in the United States alone.1 Since 2011, Cook Medical’s Hemospray has been used to treat bleeding in the GI tract, with thousands of patients treated throughout Europe, Canada and other countries around the globe.
“We are extremely pleased to receive this approval to market from FDA,” said Barry Slowey, president of Cook Winston-Salem and vice president of Cook Medical’s Endoscopy speciality. “We have worked diligently to bring a different approach to hemostasis for gastroenterology teams across the United States.”
Hemospray will soon be available to clinicians across the US for the treatment of non-variceal GI bleeds. This product is a single-use device that delivers hemostatic powder through the channel of an endoscope toward the source of a bleed. When the powder comes in contact with blood, it absorbs water and forms a gel, which acts cohesively and adhesively to create a stable mechanical barrier that covers the bleeding site. Hemospray is a nonthermal, nontraumatic treatment modality for achieving hemostasis.
“Hemospray gives clinicians another tool for the care of their patients,” said DJ Sirota, vice president of Cook Medical’s MedSurg division. “Patients have been our number one priority for over 50 years and we’ve worked hard to bring this innovation to the field of gastroenterology across the U.S.“
Numerous reports in the clinical literature have demonstrated the clinical utility of Hemospray when used alone or with other methods.2 In a summary of 19 studies of Hemospray comprising treatment of 234 patients, the combined rate of successful hemostasis was 88.5 percent. Rebleeding occurred within 72 hours in 16.2% after successful initial hemostasis with Hemospray.
Current endoscopic hemostasis treatment options can be challenging. Thermal, mechanical or contact devices can carry a risk of further tissue damage and require precise placement of the device directly onto the bleeding vessel.3 Hemospray represents a different approach to treat GI bleeds by helping to achieve hemostasis without the precision or direct visualization required of other current treatments.3 This makes Hemospray a treatment option for bleeding from damaged tissue where the bleeding source cannot be easily identified.
For more information about Hemospray and other endoscopy products, visit hemospray.cookmedical.com or cookmedical.com/endoscopy.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1 El-Tawil A., Trends on gastrointestinal bleeding and mortality: Where are we standing?, World J Gastroenterol, 2012. 18: 1154-58
2 Changela K, Papafragkakis H, Ofori E, et al. Hemostatic powder spray: a new method for managing gastrointestinal bleeding. Therapeutic Advances in Gastroenterology. 2015 May; 8(3):125-35.
3 Adverse events of upper GI endoscopy. Gastrointestinal Endoscopy, 2012. Vol. 76, No. 4: 707-708

A Bloomington Boys & Girls Club member practices with flash cards.
Bloomington, Ind. – April marked the first anniversary of Cook’s tutoring program in partnership with Bloomington’s Crestmont Boys & Girls Club. Over the past year, 30 Cook employees have volunteered more than 1,300 hours tutoring kids ages 6 to 17.
The program consists of Cook employees that volunteer to tutor students in grades K-12 at the Crestmont Boys & Girls Club. Cook employees are excused early from work twice a week to attend tutoring sessions throughout the school year. During these sessions, students receive one-on-one help with their homework and tutoring support in all subject areas.
“We discovered that we had a group of employees who were dedicated to this cause and saw an opportunity to make a bigger impact. We were able to tie donations from the company to employees’ volunteer hours,” said Pete Yonkman, president of Cook Medical and Cook Group. “We have always been committed to the communities where we live and work, and the tutoring program is a great example of how our company can support employees’ passions to make a positive impact.”
The Boys & Girls Clubs of Bloomington purchased a new building for the Crestmont Club, with renovations scheduled to be complete for the 2017-18 school year. This new building will expand the Crestmont Club from 40 to 180 students being served daily. Cook is already looking ahead to recruit another 20 employees into its tutoring program to help meet needs for the next school year.
Aaryn Eady, program director of the Crestmont Boys & Girls Club, discusses her experience with the Cook tutoring program in the video below.
5281354902001
brightcove
true
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. – Last week, Cook Medical was named Industry Partner of the Year by the Indiana Association for Adult Education for its High School Equivalency (HSE) program. In 2016, Cook partnered with Broadview Learning Center for Adults through Monroe County Community School Corporation to provide current and future employees opportunities to earn their diploma while working part-time.
“Approximately 5,000 adults in Monroe County alone do not hold a high school diploma, which is a baseline requirement for most jobs today,” said Pete Yonkman, president of Cook Medical and Cook Group. “Providing people the opportunity to earn this credential while working toward a full-time opportunity is good not only for the individuals who earn their diplomas, but also their families and the larger community surrounding them.”
Cook’s program allows individuals to work part-time at Cook while spending the remainder of the work day in class to prepare for the Test Assessing Secondary Completion. After successfully earning their HSE Diploma, these employees will be offered a full-time position at Cook.
To date, Cook’s HSE program has assisted 11 employees to obtain their HSE Diploma, and a new group of ten employees are currently enrolled. The HSE program is part of Cook’s larger education assistance program, My Cook Pathway, which provides upfront financial support to employees to pursue higher education and professional development opportunities.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. – Ivy Tech Community College Bloomington was awarded a Perkins Competitive Grant from the Indiana Department of Education in the fall of 2016 to increase rural career and technical education pathways in biotechnology. The $85,000 grant is being used to establish dual credit biotechnology courses through Ivy Tech. Starting in the spring 2017 semester at Brown County, Owen Valley, Eastern Greene and Bloomfield high schools, students have the opportunity to earn dual high school and college credit at no cost to them.
The biotechnology courses are currently taught by an Ivy Tech instructor and the grant will also provide training for high school science teachers to gain credentials to teach the coursework beginning in the 2017-18 school year. An additional $15,000 was provided to Ivy Tech Bloomington by Cook Pharmica to help launch and sustain the program for years to come by providing funding for textbooks that can be reused by the participating high schools.
“We have a mission at Ivy Tech Bloomington to help fill the local industry skills gap, and one way we do that is through partnerships like this with Cook Pharmica,” said Jennie Vaughan, chancellor at Ivy Tech Bloomington. “With the help of this grant, high school students can take dual credit classes in biotechnology, developing a clear pathway toward employment in the life sciences, an industry that’s thriving in our region.”
“We appreciate the work Ivy Tech does to help students in the local community develop the skills they need to enter the workforce upon graduating high school,” said Tedd Green, president of Cook Pharmica. “This program is a true community partnership that supports the education of our local youth and the workforce development needs of the growing life sciences industry in South Central Indiana. We are pleased to be a partner in this program.”
Since it was founded in 2004, Cook Pharmica has grown to 715 employees today. Cook also introduced its new My Cook Pathway education assistance program in 2016 to provide employees the opportunity to continue their education at no cost to them from day one with the company.
About Cook Pharmica
Cook Pharmica is a privately held contract development and manufacturing organization that provides services to the global pharmaceutical and biotech industries. Centrally located in Bloomington, Indiana, Cook Pharmica is a wholly owned subsidiary of Cook Group Incorporated. Find out more at www.cookpharmica.com.
About Ivy Tech Community College
Ivy Tech Community College (www.ivytech.edu) is the state’s largest public postsecondary institution and the nation’s largest singly accredited statewide community college system. Ivy Tech has campuses throughout Indiana. It serves as the state’s engine of workforce development, offering affordable degree programs and training that are aligned with the needs of its community along with courses and programs that transfer to other colleges and universities in Indiana. It is accredited by the Higher Learning Commission and a member of the North Central Association.




