An Interview with Cook chief medical officer Dr. John Kaufman
About 20 years ago, Cook started working on a venous valve. Our goal was to create a long-term solution for patients suffering from chronic venous insufficiency (CVI) due to reflux by creating a synthetic valve to replace the body’s natural ones. For two decades, we tested different models and gathered data, including a trial in the early 2000s.

Dr. John Kaufman, Cook’s chief medical officer
However, in December 2023, we decided to discontinue the trial. It was a tough decision. However, we feel it was the right choice, both for patients and for our company. Here’s what we learned from the experience—and here’s what we hope other life sciences companies can learn from it, too.
Can you give us some background on the goal of this trial?
Cook had been researching CVI and making solutions for it for decades. CVI affects roughly 40% of the US population, making it a huge patient population that currently doesn’t have any one-and-done cures.
Several other Cook products address some of the complications of CVI, and the valve would have fit perfectly into our portfolio of vascular venous products, such as our Zilver Vena stent, Serenity balloon, TriForce® Peripheral Crossing Set and CXI crossing catheter. However, none of those products solved the root problem. We thought a functioning valve would be the most important part of caring for these patients, so our goal was to find a percutaneous valve replacement. Cook Medical developed a valve that functions similar to the way valves the veins naturally work. The artificial valve we made was a novel proprietary design that mimicked native venous valves. To test it, we began a global, multi-site trial.
The first patient in the trial was treated in April 2023. The patient was treated by Dr. Mauricio Alviar in Barranquilla, Colombia, and the global principal investigator of the study was Dr. Paul Gagne. Our vision for the rest of the trial was that we would continue to evaluate safety, efficacy, wound healing, leg pain, and disability levels in patients over the next five years.
What outcomes did you see from the data?
As we collected data on the trial, we noticed a strange phenomenon in the clinical outcomes. Patients were reporting that they were feeling better. That’s a good thing—the only problem was that the duration of their improvement significantly outlasted the functioning of the valves. Patients were feeling better even in the absence of functioning valves. Traditional clinical outcome measures were not directly correlating to replacement valve function, raising concerns of demonstrating safety and effectiveness. Patients felt better, but the valve wasn’t functioning better.
Ultimately, we didn’t cancel this trial due to adverse events—we only canceled the trial because patients were getting better irrespective of valve function. After 20 years in this space, we firmly believe that a replacement venous valve is not a complete solution to CVI. There may be room for it to be part of a larger set of solutions for this intricate disease state. CVI is more complex than we thought, and the condition will take more than a valve to completely solve.
How did you decide to cancel the trial?
We decided to cancel the trial because the data clearly showed that the valve was not feasible and was not benefiting patients the way we had hoped it would. Without data that connects valve performance to clinical benefit, there was no way we could definitively prove the valve’s effectiveness. There was no pathway forward for approval with regulatory bodies, which pushed us to cancel the trial.
One of our Cook values is Continuous Improvement. We can’t continuously improve if we aren’t willing to change course when necessary. Twenty years of investment is a lot and it’s hard to leave. However, we prioritize patients, and if it’s not benefiting patients in a measurable way, we need to pivot.
For context, how often are clinical trials canceled?
Clinical trials are canceled more often than most people realize. In fact, only one in five clinical trials end up being completed within their original timeframe.1 About 90% of clinical drug development fails, too.2 It’s part of the innovation process.
The significant change is that the life sciences industry is starting to be more transparent about clinical trials and more willing to share knowledge. The percentage of trials with no disclosed or available information as to why the study was terminated has been decreasing steadily over the past several years, dropping from 24.1% in 2010 to 8.7% in 2021.3 In a spirit of collaboration and transparency, we are also sharing the lessons we’ve learned from canceling our venous valve clinical trial.
What did Cook learn from the clinical trial, and why are you sharing this publicly?
One of the biggest lessons we learned was that traditional outcome measures were not capturing the outcomes patients were experiencing. Traditional measures were global and encompassed the whole leg. However, these measures weren’t specific enough to capture what this one small valve was accomplishing.
We also learned that there are a lot of complexities in the venous system. Part of the struggle with this trial was that it was hard to find patients with singular pathologies with venous valve malfunction. Identifying the target population was much more difficult than expected. Most patients with venous disease have multiple comorbidities confounding the clinical benefit of an implantable venous valve, thereby limiting the target patient population. We realized that venous disease was far more complex than what a valve alone can fix, and we couldn’t enlarge the scope of the trial to cover it all.
Over the last twenty years, we’ve also learned a lot about material science. Biodesigning a valve from porcine tissue took a lot of research that will benefit us in other areas. We’ve learned about material properties that supported the valve in both animals and humans, and we can share that information.
We are sharing these learnings because research and knowledge are always valuable. Even though we discontinued the trial, the data is still valuable and can help us make better solutions in the future. We hope it will also benefit other companies that are striving to treat patients with venous conditions.
What can physicians take away from this?
If a physician is looking forward to a product in a clinical trial and then the trial is canceled, what should the physician think and feel? We understand if you’re a bit disappointed, but here are some takeaways we hope you gain.
We must challenge our own assumptions. We had made assumptions about CVI and what would cure it. Physicians also frequently need to realize the assumptions they make about disease states and treatments and be open to failure and learning from new data. Disease states can be more complicated and have more factors than previously thought; keep that in mind when treating patients. Diseases that we thought we were familiar with and thought we understood may be more complicated than we realized.
Even failures help advance knowledge. Any canceled trial can still provide more direction for future research. If you’re disappointed (as we are) about canceling a trial, there’s a silver lining that points us in the right direction for upcoming treatments.
What’s next for Cook?
Our next step is to continue to follow the patients who were treated. Although enrollment in the trial was terminated and will not be reopened, we’ll still follow patients for the planned duration of the study.
In following the industry transparency trend and in our own commitment to data transparency, we plan to publish our findings externally. Companies tend to only want to publish their positive results, but publishing negative results is important too. Cook’s unique structure as a privately owned company gives us the freedom to be open—even about the less glamorous parts of innovation.
We hope that what we have learned can benefit future efforts to help patients dealing with CVI. As we share this data, perhaps other companies will be inspired to be transparent with their data. If you are a physician, please share your personal experience treating patients with CVI. We can all learn from each other and build off publicly available data and make progress together.
Bloomington, Ind. — Cook Medical launched the next-generation EchoTip AcuCore™ Endoscopic Ultrasound (EUS) Biopsy Needle in the United States. As Cook changes to better serve our customers, we look forward to developing and offering products for a wider variety of procedures, such as AcuCore.

Cook Medical’s EchoTip AcuCore EUS Biopsy Needle
The 22-gauge AcuCore needle is used with an ultrasound endoscope for fine needle biopsies (FNB) of submucosal and extramural lesions, mediastinal masses, lymph nodes and intraperitoneal masses within or adjacent to the gastrointestinal tract. The AcuCore needle is designed with a Franseen tip made of a cobalt chromium alloy, which allows physicians to puncture tissue with flexibility, minimum force and controlled precision. To offer maximum flexibility, Cook combined the proven proprieties of the cobalt chromium with a spring-coiled sheath, coated inside and out, which enhances flexibility and makes it easier for physicians to access difficult anatomy.
“During an EUS FNB procedure, the physician relies on the needle to acquire adequate tissue sample with minimum force, especially in difficult scope positions,” said Meurisse Leahy, director of product management for Cook’s Endoscopy specialty. “The EchoTip AcuCore is a proof point of our commitment to innovating new technology and serving a wider range of patients.”

Cook Medical’s EchoTip product
In an exclusive pre-launch of the needle, Cook conducted 120 evaluations with endoscopists. Out of the 120 evaluations, 94% of physicians were satisfied and said they would use the needle again. The three attributes of the product that got the highest ratings from physicians were the needle sharpness, minimal puncture force and flexibility.
AcuCore will be on public display at the Digestive Disease Week conference in May 2024. To learn more about this product, visit the Cook booth at DDW or contact your Cook representative.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. — Cook Medical is now offering the Cook Medical’s Ascend™ Single-Use Flexible Ureteroscope in the United States and Canada. This addition is a positive change that will empower Cook to better serve more urology customers with a complete portfolio of stone management products.
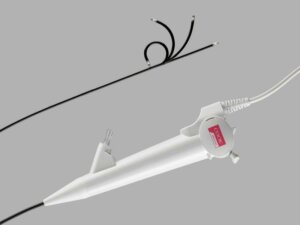
Cook Medical’s Ascend™ Single-Use Flexible Ureteroscope
“The Ascend ureteroscope makes Cook a single destination for stone management disposables. As a single-use scope, Ascend is the perfect complement to our portfolio. Physicians can complete procedures faster because they won’t have to wait for scopes to be reprocessed; Ascend is always ready,” said Johan Lowinger, director of product management for Cook’s Urology specialty. “To make this product even more accessible, Ascend is eligible for the Medicare Transitional Pass-through (TPT) payment program used by the Centers for Medicare and Medicaid Services (CMS). We are excited to make the Ascend ureteroscope, along with our other urology products, available to Cook customers.”
The Ascend scope is always ready to use and doesn’t come with the issues commonly seen with reusable ureteroscopes, such as scope fatigue, reprocessing and missing or broken parts. Ascend comes with a variety of features to help aid surgeons when performing complex urologic procedures, including features such as:
- 275 degree up/down deflection
- Deflection lock that retains the angle of deflection needed
- Standard and reverse model offering
- Enhanced illumination with transparent distal tip
To learn more about the advantages of using Ascend, visit the product page here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. — Cook Medical has been awarded an ECAT contract with the United States Department of Defense for implantable medical devices. This contract is one way Cook is being recognized for the ways we are changing to treat more patients, and we look forward to supporting those who serve our country.
“With this contract, Cook implantable devices will be available to all US DOD hospitals in the US, Hawaii and Alaska and will expand to cover all overseas hospitals in the coming months,” said Ross Harvey, vice president of supply chain at Cook. “Contracts like this one can take advantage of our global network, which helps get these products into the hands of physicians faster so Department of Defense service members can receive the medical care they need.”
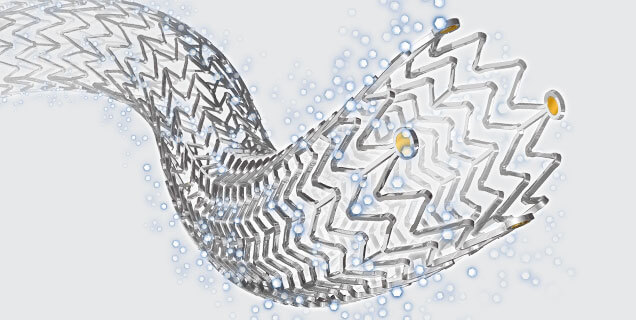
Zilver® PTX®, Cook Medical’s paclitaxel-coated stent
The contract includes implantable vascular medical devices, such as the Zilver® PTX® Drug-Eluting Peripheral Stent, Zenith® aortic endografts, and associated interventional devices to treat patients with vascular disease. The contract will streamline the process for the Department of Defense to obtain these products so they can more quickly treat military service members. The contract is for one year with four one-year extension options.
Cook has been innovating the way we work with our customers. As we form more contracts, customers gain a simplified procurement process and are able to treat more patients faster. In October 2023, Cook won a third contract with the US Department of Veterans Affairs. That contact was also for implantable devices, and it has streamlined the supply chain process.
To learn more about the benefits of working with Cook, you can contact our BusinessCare Integration (BCI) team. This team is a single point of contact for Cook’s entire product portfolio, including standard and advanced venous procedures and access technologies, simplifies contract management and order fulfillment. You can learn more about Cook BCI here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, IN and Merrimack, NH—Today, Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast® covered stent system, which recently received premarket approval for treatment of symptomatic iliac arterial occlusive disease. 
Cook Medical will assume sales, marketing and distribution rights for the product in the United States over the coming months. The iCast covered stent system will continue to be manufactured by Atrium Medical Corporation in Merrimack, NH, which is part of Getinge.
“This agreement with Cook Medical ensures that the iCast covered stent system will reach the optimum number of patients who will benefit from it in the United States,” said Patricia Fitch, president of Getinge NA. “This distribution agreement allows Getinge to best serve our U.S. market and accelerate our product innovation pipeline, which is in alignment with our global business strategy.”
“This product fills the need of a covered stent in our vascular portfolio with a proven technology. iCast has five-year data aligned with our commitment to long-term clinical evidence and predictable results for PAD Therapies,” said Mark Breedlove, senior vice president of Cook Medical’s Vascular division. “Our team is excited to support and distribute the iCast covered stent system across the U.S.”
Cook and Getinge continue to collaborate in other strategic areas, also partnering to complete the PRESERVE-Zenith® Branch Endovascular Graft-Iliac Bifurcation IDE study with five-year follow up. The purpose of this study was to evaluate the safety and effectiveness of the Zenith Branch Endovascular Graft-Iliac Bifurcation in combination with the Atrium iCast covered stent in patients requiring treatment of aortoiliac and iliac aneurysms. Additional information about the clinical study is available at clinicaltrials.gov (NCT02571907).
ICast is a registered trademark of Atrium Medical Corporation.
About Getinge
Getinge is a global provider of innovative solutions for operating rooms, intensive-care units, sterilization departments and for life science companies and institutions. Based on our first-hand experience and close partnerships with clinical experts, healthcare professionals and medtech specialists, we are improving the everyday life for people, today and tomorrow.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
News coverage of this announcement
Endovascular Today—Cook Medical Will Distribute Getinge’s iCast Covered Stent System in the United States
Vascular News—Getinge and Cook Medical enter US commercial distribution agreement for iCast covered stent
Mass Device/Medical Tubing and Extrusion—Getinge, Cook Medical sign U.S. commercial distribution agreement for iCast covered stent
Vascular Specialist—Getinge and Cook Medical enter US commercial distribution agreement for iCast covered stent
Interventional News—Getinge and Cook Medical enter US commercial distribution agreement for iCast covered stent
Vascular Disease Management—Getinge and Cook Medical Enter Into Commercial Distribution Agreement for iCast Covered Stent System in the United States
Getinge—Getinge enters into commercial distribution agreement with Cook Medical for iCast covered stent system in the United States
Bloomington, Ind. — Today, Cook Medical and Bentley announced a United States distribution agreement for the BeBack Catheter. Over the coming months, Cook Medical will be assuming commercial responsibilities for this Bentley product.
“We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” said Alec Cerchiari, director of product management for Cook’s PAD and Venous specialty. “The BeBack Catheter is a unique tool for crossing heavily calcified lesions in antegrade or retrograde fashion. It complements Cook’s robust portfolio of above- and below-the-knee products, including our Micropuncture® Pedal Introducer Access Sets, Flexor® Guiding Sheaths and CXI® Support Catheter. ”
The BeBack is a crossing catheter designed for steering through chronic total occlusions (CTO) that provides targeted reentry options. The catheter includes features such as:
- A steerable and adjustable nitinol needle
- A radiopaque marker located near the tip of the catheter, which indicates the direction in which the needle curves
- 80 and 120 cm lengths and 2.9F and 4F sizes

Cook is distributing Bentley’s BeBack Catheter.
“The BeBack catheter is an effective approach for successful recanalisation and should be available in every lab across the U.S. Thanks to this collaboration with Cook, we will now have a better commercial footprint in the U.S. with even more opportunities to treat patients with debilitating arterial and venous disease,” said Martijn Nugteren, director of sales & marketing at Bentley.
Distribution partnerships, such as this one with Bentley, allow Cook to accelerate our product pipeline and bring innovative products to our customers and their patients. Cook and Bentley have a long history of collaboration, and this partnership represents another example of us working together to advance patient care.
You can learn more about the BeBack® product here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
About Bentley
Founded in 2009 by Lars Sunnanväder and Miko Obradovic, Bentley InnoMed GmbH is based in the medical technology stronghold of Hechingen in Baden-Wurttemberg, Germany. The company develops, manufactures, and markets implants and catheters for the endovascular treatment of peripheral vascular and aortic diseases. The company has since then become a leading global manufacturer and the European market leader for covered stents. The company is part of the Bentley Endovascular Group. More than 400 employees are committed to the Group’s strong culture of innovation, making Bentley one of the most important employers in the county of Zollernalb and beyond. In 2022, the company generated sales revenue of EUR 69 million. Based on its product innovations and the development of new markets, the company is on a path of strong growth momentum.
News coverage of this announcement
Endovascular Today—Cook Medical to Distribute Bentley’s BeBack Catheter in the United States
Cath Lab Digest—Cook Medical and Bentley Enter Distribution Agreement for the BeBack Catheter in the United States
Vascular News—Bentley and Cook Medical enter US distribution agreement for BeBack crossing catheter
SVS Vascular Specialist—Bentley, Cook Medical enter US distribution agreement for BeBack device
Bloomington, Ind. — Cook Medical’s shorter Liver Access and Biopsy Set (LABS) is back on the US market with a new pediatric indication. Originally indicated for use in adults only, the set now has FDA clearance for use in adolescents, children and infants. The expanded indication for LABS is another proof point of Cook’s ongoing commitment to providing innovative support to vulnerable patient populations. 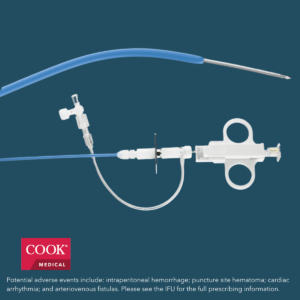
The LABS pediatric indication follows last year’s establishment of the Crossroads Pediatric Research Consortium, an alliance that includes Cook Medical, the Indiana University School of Medicine and Purdue University’s Department of Biomedical Engineering. The consortium is focused on meeting unmet needs for pediatric patients by accelerating the development, approval and availability of innovative medical devices for children. Cook is proud to invest in pediatric research and will continue innovating for this important patient population.
LABS is indicated for use in obtaining liver histology samples via jugular vein approach. All LABS sets contain compatible components for transjugular liver access and tissue biopsy, including:
- A precurved sheath and cannula to better access the hepatic vein
- Quick-Core® Biopsy Needle
- Check-Flo Performer® Assembly
- Accessory catheters
“Our pediatric LABS has shorter versions of the original medical devices to more appropriately fit pediatric patients’ smaller bodies,” explained Remco van der Meel, director of product management for Cook’s Interventional specialty. “Now, LABS will help physicians diagnose an array of conditions in a wider variety of patients. It’s an extremely versatile set of products that can now treat even the smallest patients.”
To learn more about Cook’s LABS products, visit the product page.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
Bloomington, Ind. and Belgium — Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specializing in catheter securement devices marketed under the brand FlexGRIP®. FlexGRIP devices are now available as an addition to Cook’s comprehensive portfolio of percutaneous drainage products to improve patient comfort and experience during treatment.
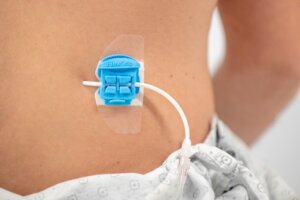
The Bedal FlexGRIP catheter securement device is now available with Cook products.
As a result of the collaboration, FlexGRIP catheter securement devices are now available to Cook customers in Europe, Canada and the United States. Using this well-established device helps reduce the risk of dislodgment, infection and kinking of the catheter. Bedal’s proprietary air-pillow technology ensures highly secure catheter stabilization, in addition to comfort and ease of use, and allows the patient more freedom of movement.
Alexander Van Damme, CEO of Bedal International, expressed excitement about the partnership, stating, “We are thrilled to partner with Cook Medical to bring the FlexGRIP catheter securement device worldwide. This collaboration represents a synergistic union of expertise, innovation and commitment to advancing medical solutions.”
“When patients are treated with Cook catheters, we think about the patient’s whole experience. That means not just the outcome—it also means we think about how comfortable the patient is during treatment,” said Remco van der Meel, director of product management for Cook’s Interventional specialty. “The FlexGRIP device is the perfect complement to Cook’s comprehensive portfolio of percutaneous drainage products. This well-designed and secure fixation device will help promote better outcomes and improve patient experiences, and we’re excited to have this product as an option available to all Cook customers who are using our percutaneous drainage products.”
To learn more about the benefits of adding FlexGRIP to Cook’s products, visit the product page.
FlexGRIP is a registered trademark of Bedal International.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
About Bedal International
Bedal International, a dynamic scale-up in the medical device industry, focuses on specialized catheter securement solutions. The FlexGRIP>sup>® products prioritize patient well-being and healthcare provider convenience. Dedicated to innovation, Bedal International strives to enhance the overall quality of patient care. Explore more at www.flex-grip.com.








