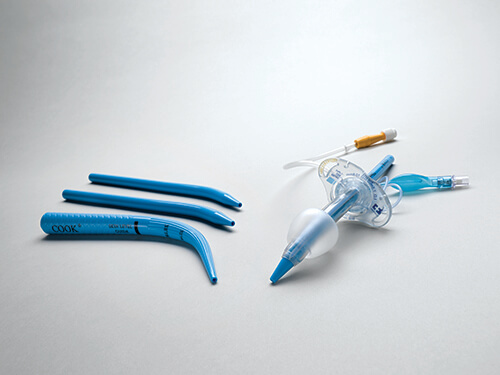
Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino product family and is designed to fit a wide range of tracheostomy tube sizes.
For more than 30 years, Cook has been a pioneer when it comes to innovating percutaneous dilational tracheostomy and airway management products. The predecessor product of Blue Rhino, a tracheostomy introducer set developed with Dr. Pasquale Ciaglia, included a series of dilators intended to be used sequentially to create an opening in the airway so that a tracheostomy tube could be inserted. Later, the introducer set was consolidated into a single product now known as the Blue Rhino. The grips and grooves on the product’s unique design were developed with Dr. Dietmar Enk. Today’s Blue Rhino G2-Multi introducer dilator is shaped with a gradual taper for single-stage dilation. The product comes in a variety of kit configurations to accomodate physicians’ preferences, enabling them to perform tracheostomies at the bedside in the ICU.
The new loading dilators in the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays have been designed to fit with Medtronic’s newest line of tracheostomy tubes for most adult sizes: the Shiley™ Adult Flexible Tracheostomy Tube with TaperGuard™ Cuff, Disposable Inner Cannula and Shiley™ Adult Flexible Evac Tracheostomy Tube with TaperGuard™ Cuff and subglottic secretion management. Each set and tray comes with a range of loading dilators to help physicians find the right fit for the patient.
“Cook Medical has seen a significant increase in demand for our Blue Rhino products since the onset of the COVID-19 pandemic,” said Andy Cron, vice president for Cook’s Critical Care business. “Tracheostomies are performed on patients who need long-term ventilation, such as those suffering from acute respiratory failure. Percutaneous tracheostomies are associated with fewer bleeding complications.1 The Blue Rhino can be used at the patient’s bedside, eliminating the need to transport these critically ill patients to the operating room for a surgical procedure.”
“Even after 30 years of offering this product, we are still looking for ways to innovate and make our patients’ experiences better,” said Cron. “This is an example of Cook listening to patient and physician feedback and creating a new version that is compatible with more tracheostomy tubes.”
For information about Cook’s Vista educational courses on percutaneous tracheostomy procedures using the Blue Rhino Multi Introducer, see our Vista training availability. To learn more about the product, visit CookMedical.com/BlueRhinoMulti.
1 Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care 2006;10(2):R55-R55.
About Cook Medical
Since 1963, Cook Medical has been inventing, manufacturing and delivering a unique portfolio of medical devices to healthcare systems around the world. We work closely with physicians to develop technologies that improve patients’ lives. Because we remain family owned, we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at CookMedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.