Bloomington, Ind. — Following the publication of an animal study examining the performance of embolization coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of JVIR, examined fibered and nonfibered embolization coil performance, this time in the venous system.
In this newly published venous ovine study, fibered Nester® coils and nonfibered coils were deployed in 24 veins in 6 sheep. The study’s data confirmed that fibered coils demonstrate several advantages over nonfibered coils. These advantages include the following:
- Fibers significantly boosted the immediate occlusive capacity of coils. In the study, fibered coils achieved stasis in 5.3 minutes, while nonfibered coils took 9.0 minutes.2
- A significantly lower average radiation dose was required when fibered coils were used: 25.3 mGy for fibered coils compared with 34.9 mGy for nonfibered coils.2
The venous fiber study also substantiated the findings of the earlier arterial fiber study: The two studies found that fewer fibered coils are needed to achieve acute occlusion compared to bare metal coils.1, 2 Both studies confirmed that significantly less coil length is needed if the coil is fibered.1, 2
- In the venous fiber study, on average 5 fibered coils, or 70 cm of fibered coil length, were needed to achieve occlusion compared with 8.75 nonfibered coils, or 122.5 cm of bare metal coil length.2
- In the arterial fiber study,on average 1.3 fibered coils, or 9.1 cm of coil length, were required to achieve occlusion, compared to 2 nonfibered coils, or 22.4 cm of nonfibered coil length, that was needed to obtain occlusion.1
Should the study results translate to the human experience, patients treated with fibered coils could potentially experience shorter procedure times, require fewer implants and have less radiation exposure to achieve the same outcomes.*
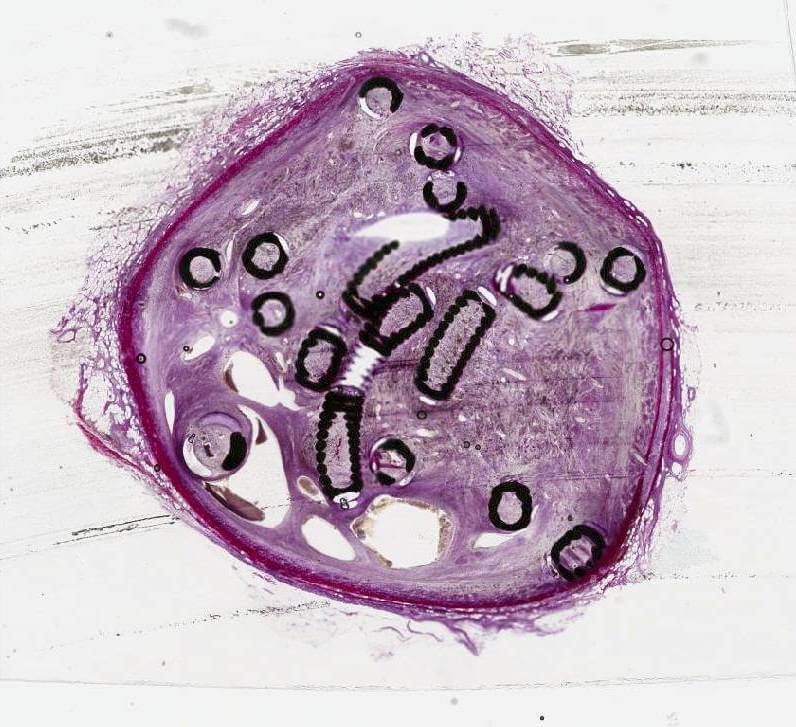
This study used a unique method of generating histologic images of metallic implants by cutting 5µm thin sections of tissue embedded in resin.
In this venous fiber study, a new approach was also applied to generating histologic images. A unique method of cutting 5 µm thin sections of tissue with metallic implants was developed by Cook Research Incorporated (CRI) and Alizée Pathology. Thin sectioning results in clearer and more detailed histologic images, allowing a pathologist to read and interpret tissue changes. An image showing a 5 µm thick microscope slide of Cook embolization coils in an ovine vein can be seen on the front cover of the May 2023 issue of JVIR and online at www.jvir.org.
“When we see in vivo results like this in studies, we hope the same benefits will be applicable to human patients,” said Remco van der Meel, director of product management for Cook’s Interventional specialty. “We are constantly evaluating our Embolization portfolio, exploring cutting-edge technology and gathering data to create new technology. Additionally we want to understand how we could best support our customers in achieving the best possible outcomes for patients. This study helps us make data-based decisions on how to best meet the needs for effective embolization in a cost-efficient manner, while reducing exposure to ionizing radiation for both doctor and patient. These are key elements in today’s healthcare environment.”
To read the full venous animal fiber study, view the article on JVIR’s website. To read the full arterial fiber study article, go here. To learn more about fibered coils, visit our Cook Medical embolization web page here.
*Definitive conclusions regarding the safety or effectiveness of fibered coils in humans cannot be directly drawn from the results of these animal studies.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
News coverage of this announcement
Endovascular Today—Cook Medical’s Nester Fibered Coils Evaluated for Venous Use in Animal Study
Vascular Disease Management—Second Cook-Sponsored Animal Study Showcases Unique Imaging Methodology and Reveals New Data on Fibered vs Nonfibered Embolization Coils
Venous News—Study finds fibred embolisation coils reduce procedure times and radiation exposure
1 Trerotola SO, Pressler GA, Premanandan C. Nylon fibered versus non-fibered embolization coils: comparison in a swine model. J Vasc Interv Radiol. 2019;30(6):949–955.
2 White SB, Wissing ER, Van Alstine WG, et al. Comparison of fibered versus nonfibered coils for venous embolization in an ovine model. J Vasc Interv Radiol. 2023;34(5):888–895.
