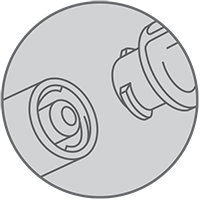
ENFit
Cook Medical is committed to enhancing patient safety by reducing potentially harmful misconnections between medical devices. For this reason, Cook and other medical device manufacturers around the world are transitioning to new international (ISO) standards for small-bore connectors in healthcare devices, starting with enteral feeding connectors. Cook is collaborating with other medical device manufacturers to adopt the ENFit connection, which requires a twisting motion in order to connect, on its feeding tubes. The two ends of the ENFit connection are designed to fit only enteral devices. The ENFit connection meets the new ISO design standard.
Entuit® Gastrostomy BR Balloon Retention Feeding Tube with ENFit connection
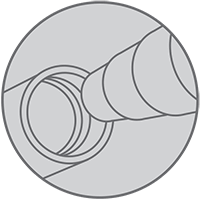
Non-ENFit
Hospitals and medical device companies are in the process of transitioning to the permanent ENFit connection. This will affect syringes, administration sets, and enteral feeding tubes. Because every hospital is on a unique timeline for this transition, you may not have a device that is ENFit-compatible yet. During the transition, Cook will work to ensure you have access to what you need, including information on how to use and care for your Cook non-ENFit feeding tube.
Entuit Gastrostomy BR Balloon Retention Feeding Tube