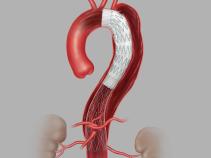
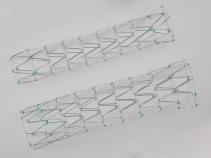
The Zenith Dissection Endovascular System (Zenith TX2 Dissection Endovascular Graft with Pro-Form and Zenith Dissection Endovascular Stent) is indicated for the endovascular treatment of patients with Type B aortic dissection. The Zenith TX2 Dissection Endovascular Graft with Pro-Form is intended to seal the entry tear(s) and to exclude aneurysms associated with chronic dissections. The Zenith Dissection Endovascular Stent is intended to be used as a distal component to provide support to delaminated segments of non-aneurysmal aorta with dissection distal to a Zenith TX2 Dissection Endovascular Graft with Pro-Form. The system is indicated for use in patients having suitable vascular anatomy for endovascular repair, including:
• Adequate iliac/femoral access compatible with the required introduction systems
• For the Zenith TX2 Dissection Endovascular Graft with Pro-Form:
o Non-dissected/aneurysmal aortic segments (fixation sites) distal to the left common carotid artery and proximal to the entry tear with a length of at least 20 mm,
o Non-dissected/aneurysmal aortic segments (fixation sites) distal to the left common carotid artery and proximal to the entry tear with a diameter (measured outer-wall to outer-wall) of no greater than 38 mm and no less than 20 mm, and
• For the Zenith Dissection Endovascular Stent:
o Diameter at non-aneurysmal intended implant site for the stent (measured outer-wall to outer-wall) of no greater than 38 mm (true lumen) and no less than 20 mm (total aortic diameter).
The products on this website are available for sale in the United States. Reference Part Numbers are not the same in all countries/regions. For products available in other countries, please choose one of our other websites from the region selector at the top of the site or contact your local Cook Medical representative.
Specifications
| Order Number | Reference Part Number |
Instructions for Use (IFU) |
MR Status |
Aortic Vessel OD mm |
Diameter mm |
Length mm |
More Info |
|---|---|---|---|---|---|---|---|
| 36 mm Diameter | |||||||
| G47491 | ZDES-36-80-US |
|

|
20-30 | 36 | 80 | Expand » |
|
Additional Specs
Description
-
Stents
4
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||
| G47492 | ZDES- 36-120-US |
|

|
20-30 | 36 | 120 | Expand » |
|
Additional Specs
Description
-
Stents
6
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||
| G47493 | ZDES-36-180-US |
|

|
20-30 | 36 | 180 | Expand » |
|
Additional Specs
Description
-
Stents
9
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||
| 46 mm Diameter | |||||||
| G47494 | ZDES-46-80-US |
|

|
31-38 | 46 | 80 | Expand » |
|
Additional Specs
Description
-
Stents
4
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||
| G47495 | ZDES-46-120-US |
|

|
31-38 | 46 | 120 | Expand » |
|
Additional Specs
Description
-
Stents
6
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||
| G47496 | ZDES-46-185-US |
|

|
31-38 | 46 | 185 | Expand » |
|
Additional Specs
Description
-
Stents
9
Introducer Sheath ID/OD Fr (mm)/mm
16 (5.3)/6.0
|
|||||||