Over the last 60 years, the flexible endoscope has been instrumental for surgeons as therapeutic intervention has evolved. Today, surgeons are going beyond the traditional exploratory boundaries of endoscopic surgery to deliver treatment.
Read this special report from General Surgery News about the rising trend of therapeutic endoscopy.
At Cook Medical, we’re proud to play a part in advancing treatment. Check out our expanding product portfolio in therapeutic endoscopy:
 Hemospray® Endoscopic Hemostat
Hemospray® Endoscopic Hemostat
Used for hemostasis of nonvariceal gastrointestinal bleeding. For use only with a 2.8 mm or larger flexible endoscope within the GI tract.
Summary of Clinical Data: Clinical data summary information that was, in part, the basis for granting the de novo can be found on the Cook Medical website at CookMedical.com/HemosprayData
 Instinct Plus™ Endoscopic Clipping Device
Instinct Plus™ Endoscopic Clipping DeviceUsed for endoscopic clip placement within the gastrointestinal tract for the purposes of: 1) endoscopic marking, 2) hemostasis for mucosal and submucosal defects less than 3 cm, bleeding ulcers, arteries less than 2 mm, polyps less than 1.5 cm in diameter, diverticula in the colon, and prophylactic clipping to reduce the risk of delayed bleeding post lesion resection, 3) anchoring to affix jejunal feeding tubes to the wall of the small bowel, 4) as a supplemental method for closure of GI tract luminal perforations less than 20 mm that can be treated conservatively, and 5) anchoring to affix fully covered esophageal self-expanding metal stents to the wall of the esophagus in patients with fistulas, leaks, perforations, or disunion.
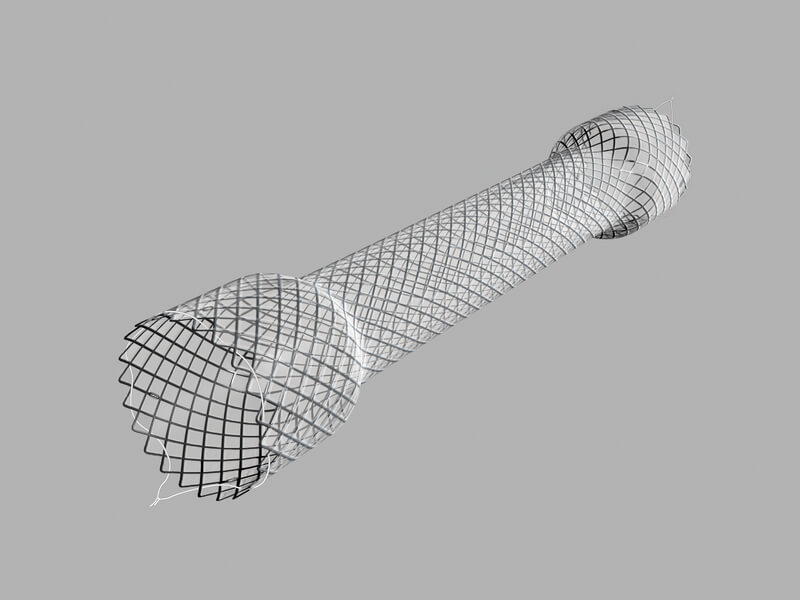 Evolution® Esophageal Controlled-Release Stent – Fully Covered
Evolution® Esophageal Controlled-Release Stent – Fully Covered
Used to maintain patency of malignant esophageal strictures and/or to seal tracheoesophageal fistulas.
 Evolution® Esophageal Controlled-Release Stent – Partially Covered
Evolution® Esophageal Controlled-Release Stent – Partially Covered
Used to maintain patency of malignant esophageal strictures and/or to seal tracheoesophageal fistulas.
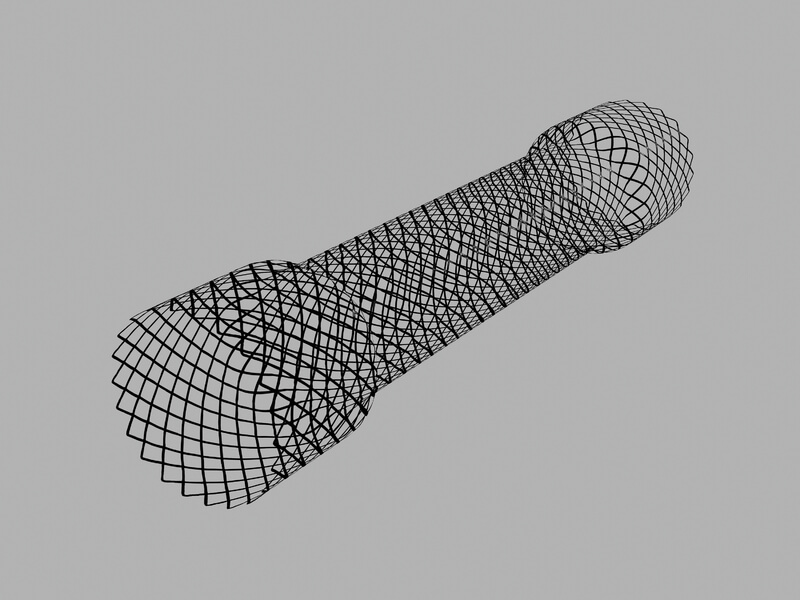 Evolution® Duodenal Controlled-Release Stent – Uncovered
Evolution® Duodenal Controlled-Release Stent – Uncovered
Used for palliative treatment of duodenal or gastric outlet obstruction or strictures caused by malignant neoplasms.
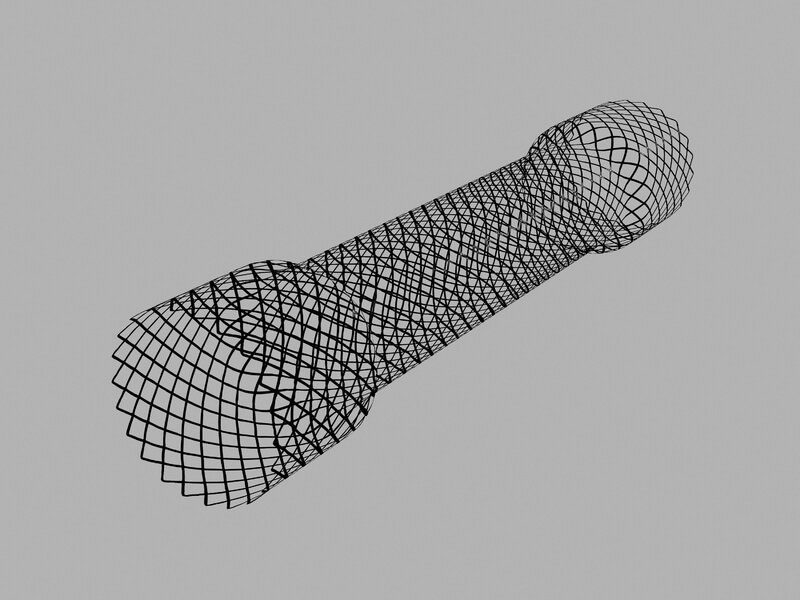 Evolution® Colonic Controlled-Release Stent – Uncovered
Evolution® Colonic Controlled-Release Stent – Uncovered
Used for palliative treatment of colonic obstruction or colonic strictures caused by malignant neoplasms, and to relieve large bowel obstruction prior to colectomy in patients with malignant strictures.
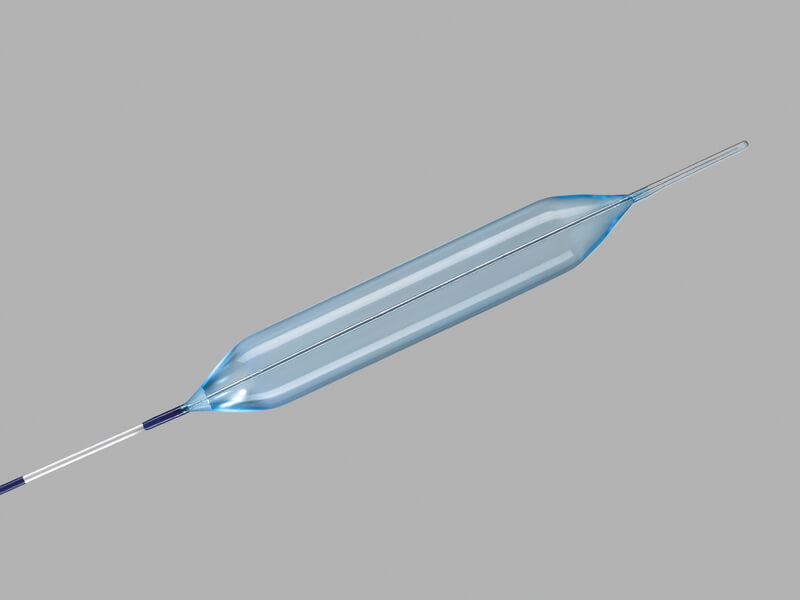 Hercules® 3 Stage Balloon
Hercules® 3 Stage Balloon
Used to endoscopically dilate strictures of the esophagus.
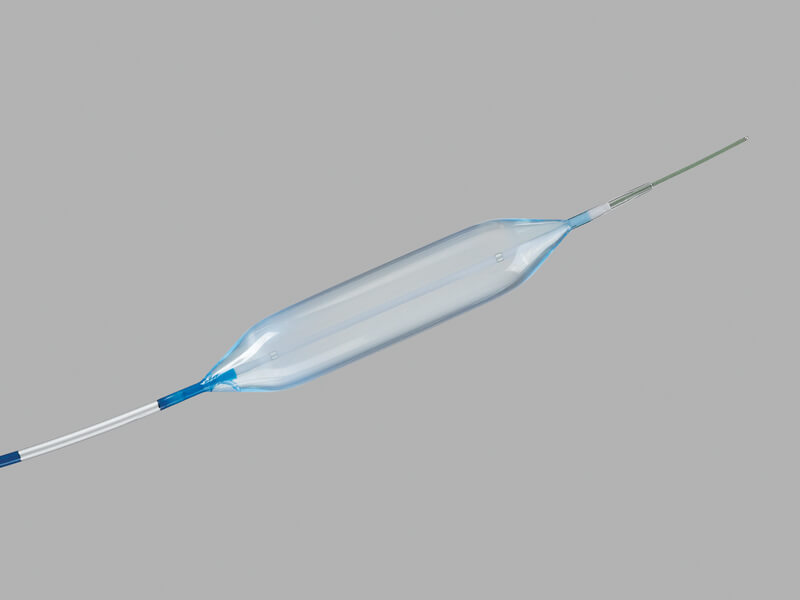 Hercules® 3 Stage Wire Guided Balloon
Hercules® 3 Stage Wire Guided Balloon
Used to endoscopically dilate strictures of the gastrointestinal tract including the esophagus, pylorus, duodenum, and colon.



