Collaboration is in our DNA. For 50 years and counting, Cook Medical has partnered with urologists around the world to advance their procedures with innovative products that better meet their patients’ needs. From working with physicians to bring their product ideas to life, to industry partnerships like the one we have with Quanta System to distribute the most advanced laser technologies, Cook Medical continues to help shape the urological landscape.
Putting patients—whether young or old—first comes first for us, and we’re proud to offer a selection of products specifically indicated and sized for children.
Our legacy of innovation lives on, and we continue to take a holistic “One Cook” approach—with one vision and one purpose—to deliver cost savings, a streamlined supply chain, and product innovation every step of the way. As the industry evolves, we continue to look for new ways to support the changing landscape and advance Urology.
Here’s to another 50 years and beyond.
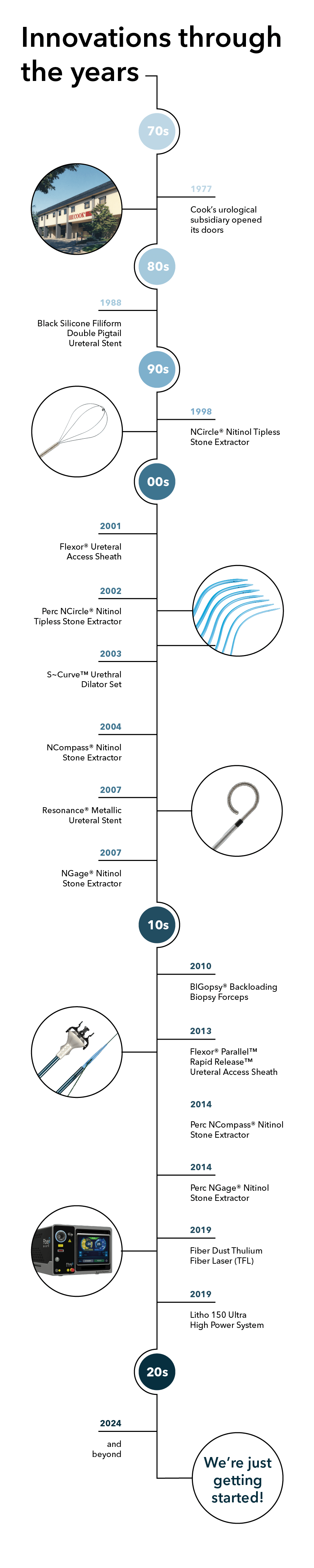
Take a closer look at some of our innovative milestones through the years:
1970s
In 1974, our founder Bill Cook was introduced to Dr. Alvin Rutner. Considered a urological pioneer, Dr. Rutner had some drawings and notes on a few product ideas that he shared with Cook, establishing a relationship that led to numerous cuttingedge product innovations:

Sparked by Dr. Rutner’s ideas, it quickly became obvious that there was real potential for Cook Medical to grow by serving the urological community. In September 1977, Cook’s urological subsidiary—Vance Products Incorporated (VPI)—opened its doors in a refurbished warehouse in downtown Spencer, Indiana. Eventually VPI would change its name to Cook Urological Incorporated, and the rest is history.
1980s
Stent innovation is foundational for us, and the Black Silicone Filiform Double Pigtail Ureteral Stent (1988) is a stent that has been making a difference for countless patients—including those with stent pain concerns1 and those with a history of encrustation.2
1990s
With its unique truly tipless design, the NCircle® Nitinol Tipless Stone Extractor (1998) was the first basket to feature nitinol wire construction.3 The resilient, memory characteristics of the nitinol wire allow the basket to return to its original shape when the device is deployed. The tipless configuration allows the physician to position the basket directly against the mucosal lining to minimize trauma, reduce bleeding, and maintain a clear working field. Also available in Delta Wire® and Helical options.
2000s
The Flexor® Ureteral Access Sheath (2001) was one of the first devices on the market to feature reinforced coil construction to help maintain a working channel and facilitate the passage of endoscopes and other instruments into the urinary tract.
We expanded our nitinol extractor offering with the Perc NCircle® Nitinol Tipless Stone Extractor (2002), an option specifically designed for percutaneous nephrolithotomy (PCNL), and ultimately revolutionized percutaneous stone removal.
Another product of physician collaboration, the dilators within the S~Curve™ Urethral Dilator Set (2003) are specifically designed to navigate the natural curvature of the male urethra to dilate male urethral strictures and vesical neck contractures.
The unique multiwire geometry of the NCompass® Nitinol Stone Extractor (2004) provides a traditional 3 or 4 wire basket design at full deployment, while partial closure reconfigures the basket to provide the exceptionally tight weave of a 12 or 16 wire basket. At full deployment, the distal portion produces a netting effect that can help you enclose smaller fragments and prevent stone migration.
And then there’s the NGage® Nitinol Stone Extractor (2007), which has proven to be a game-changer with its innovative hybrid basket and grasper design to directly target a stone and secure it for removal or reposition and release it to be fragmented.
Today, Cook Medical continues to be the recognized leader in nitinol stone extractor technology.
Another novel stent innovation, the Resonance® Metallic Ureteral Stent (2007) features a coiled-wire construction that helps maintain urine flow even when the stent is under severe extrinsic compression. As a result, the Resonance stent is significantly more resistant to external compression than traditional polymer stents.4 In addition, the Resonance stent’s radial strength allows it to remain indwelling for up to 12 months. This relatively long indwelling time minimizes the need for multiple stent placements and the complications that are associated with frequent stent exchange.
2010s
Used to obtain tissue samples for pathological evaluation, the 4 mm³ cup size of the BIGopsy® Backloading Biopsy Forceps (2010) provides a sample four times larger than samples from comparable sized models. This increases the likelihood of obtaining a representative tissue sample for the pathologist without costly repeated procedures.
With the addition of the Flexor® Parallel™ Rapid Release™ Ureteral Access Sheath (2013), the Flexor family expanded to give physicians the option to use a single wire guide as both a safety and working wire using the rapid release technique.
Mimicking the extraction characteristics of their URS predecessors, we added two more nitinol devices in 2014, with specific indications for percutaneous stone removal: the Perc NCompass® Nitinol Stone Extractor and Perc NGage® Nitinol Stone Extractor.
In 2019, we partnered with Quanta System to deliver the most advanced laser systems, like the Fiber Dust Thulium Fiber Laser (TFL) and a selection of holmium lasers led by the Litho 150 Ultra High Power System. And with Quanta, innovative pulse technology like Virtual Basket comes standard.
Read more about our pioneering partnership with Quanta here.
2020s and beyond
We’re just getting started!
- Gadzhiev N, Gorelov D, Malkhasyan V, et al. Comparison of silicone versus polyurethane ureteral stents: a prospective controlled study. BMC Urology. 2020;20(1):10.
- Watterson JD, Cadieux PA, Stickler D, et al. Swarming of proteus mirabilis over ureteral stents: a comparative assessment. J Endourol. 2003;17(7):523-527.
- Monga M. Ureteroscopy: Indications, instrumentation & technique. New York: Humana Press; 2013:180.
- Polcari AJ, Hugen CM, López-Huertas HL, et al. Cost analysis and clinical applicability of the Resonance Metallic Ureteral Stent. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):11-15.