Pioneers of peripheral medicine
Cook has worked alongside the pioneers of peripheral medicine for 60 years and remains dedicated to providing therapies and technologies that treat peripheral arterial and venous diseases. Globally, these diseases impact millions of patients and require specialized devices and treatment options to improve patients’ quality of life.
Peripheral arterial disease (PAD) often occurs in the legs and can lead to amputation; those with the disease have a greater risk of suffering a heart attack or stroke.1 Zilver® PTX® is the only drug-eluting stent with a superficial femoral artery trial that shows superior 5-year results compared to standard of care.2 Because PAD below the knee (BTK) can be challenging to treat, our goal is to serve as a procedural partner in treating this disease. We have designed devices to increase efficiency, improve outcomes, and preserve limbs.
Venous disease is complex and can impact patients of younger and more diverse backgrounds than other vascular diseases. We are committed to helping all patients affected by deep venous disease by building awareness of the symptoms that can progress to pulmonary embolism or venous obstruction and developing therapies to minimize disease progression.
Cook delivers a comprehensive offering of introducer sets, wire guides, sheaths, catheters, balloons, and stents that enable physicians to effectively access, target, and treat these diseases. We offer a full line of filters and retrieval sets for the prevention of recurrent pulmonary embolism (PE).
- Facts about peripheral arterial disease (PAD). National Heart, Lung, and Blood Institute. NIH Publication No. 21-HL-5837; December 2021.
- Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016; 133(15):1472–1483.
Cook Medical’s Zilver Vena Venous Self-Expanding Stent has shown high rates of sustained patency as well as freedom from clinically driven intervention over 3 years, according to recently published data in the Journal of Vascular and Interventional Radiology (JVIR). This…

While the treatment of venous obstruction has seen significant advancements in recent years, there continues to be a lack of robust evidence or standardized best practices for venous specialists to follow. This has been particularly challenging for those managing lower…
Study suggests effective treatment of symptomatic iliofemoral venous outflow obstruction1 The study, “Twelve-month end point results from the evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study),” has shown that…
An interview with Dr. Eric Secemsky Eric A. Secemsky, MD, MSc, RPVI, FACC, FAHA, FSCAI, FSVM Director of Vascular Intervention, Beth Israel Deaconess Medical Center Section Head, Interventional Cardiology and Vascular Research, Smith Center for Outcomes…
Watch videos below while the experts talk about treatment trends, product features, and usage “If I want to just get some good distal flow and I want to get into those tibials, the Serenity balloon is my mainstay.” …
On July 11, 2023, the FDA sent out a letter to healthcare providers titled, “UPDATE: Paclitaxel-Coated Devices to Treat Peripheral Arterial Disease Unlikely to Increase Risk of Mortality- Letter to Health Care Providers.” The FDA has now determined the data…
Cook’s 2nd generation CXI Support Catheter has earned its reputation as a premium technology for your procedural essentials in Peripheral Arterial Disease (PAD) cases. Cook Medical launched the new 2.6 Fr catheter on March 25, 2019, in the U.S. and…
With a new 5 mm diameter stent added to the Zilver PTX arsenal, Cook Medical has expanded its offering of the broadest range of drug-eluting stent (DES) sizes available to treat peripheral arterial disease (PAD) in the superficial femoral artery…
Peripheral arterial disease (PAD) is a serious condition that affects 8-12 million Americans each year.1 PAD occurs when fatty deposits form in arteries that are outside of your heart. These fatty deposits (or plaque) can restrict the flow of blood.…
On March 25, Cook Medical launched the new 2.6 Fr CXI® Support Catheter with platinum-iridium marker bands. The new 2.6 Fr CXI, which is now available in the US and Canada, comes with additional tip configurations and a new 135…
On Dec. 6, Dr. Konstantinos Katsanos and his coauthors created tremendous controversy when they published a meta-analysis on paclitaxel-coated balloons and stents in the Journal of the American Heart Association. Using data from 28 randomized controlled trials (RCT), the authors…
Zilver® PTX® global data analysis reveals proven long-term benefit, real-world outcomes1 On Nov. 6, Dr. Michael Dake, senior vice president for University of Arizona Health Sciences, presented results from a global data analysis on Zilver PTX at VIVA (Vascular InterVentional…
Cook Medical is excited to invite you to attend a talk during the PAD Luncheon at VIVA 2018. Title: “The first DES gets the last word: unmatched evidence supports the first FDA-approved SFA drug-eluting stent” Topic covered: Understanding the basics of…
Visit our booth, #400, and come chat with us in our Cook Vascular Suite in Lafite 1. And we cordially invite you to attend our AORTIC Armageddon Luncheon and PAD Luncheon talks. AORTIC Armageddon Luncheon Title: “Achieving durable endovascular aortic…

Cook Medical is excited for VEINS at VIVA 2018. We hope you’ll visit our booth to learn what we’re up to in the world of venous endovascular interventional strategies. Stop by to chat with our staff and discuss all things…

The best medical device technology is only as good as a customer’s ability to use it safely and effectively. Usability testing is the key to ensuring medical devices are…
 While the treatment of venous obstruction has seen significant advancements in recent years, there continues to be a lack of robust evidence or standardized best practices for venous specialists to follow. This has been particularly challenging for those managing lower…
While the treatment of venous obstruction has seen significant advancements in recent years, there continues to be a lack of robust evidence or standardized best practices for venous specialists to follow. This has been particularly challenging for those managing lower…  Cook Medical is excited for VEINS at VIVA 2018. We hope you’ll visit our booth to learn what we’re up to in the world of venous endovascular interventional strategies. Stop by to chat with our staff and discuss all things…
Cook Medical is excited for VEINS at VIVA 2018. We hope you’ll visit our booth to learn what we’re up to in the world of venous endovascular interventional strategies. Stop by to chat with our staff and discuss all things…  NEW lunch sessions! Get lunch and grab a seat. Listen to clinical experts engaging in lively debate on the hottest topics in the vascular world. Pardon the Intervention will take place Tuesday-Friday, from 12 noon to 1 pm, in the…
NEW lunch sessions! Get lunch and grab a seat. Listen to clinical experts engaging in lively debate on the hottest topics in the vascular world. Pardon the Intervention will take place Tuesday-Friday, from 12 noon to 1 pm, in the…  Get closer to the action. Our new 3rd floor pavilion is just outside the Grand Ballroom East/West in the promenade. (Click to enlarge) Steal away for a minute. NEW for 2017! We’re turning the 2nd floor Sutton Parlor North into…
Get closer to the action. Our new 3rd floor pavilion is just outside the Grand Ballroom East/West in the promenade. (Click to enlarge) Steal away for a minute. NEW for 2017! We’re turning the 2nd floor Sutton Parlor North into… 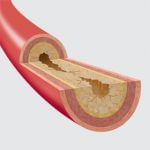 September is Peripheral Artery Disease (PAD) Awareness Month. According to the CardioVascular Coalition, PAD affects approximately 18 million US citizens, and of those, an estimated 160,000 to 180,000 will have a limb amputated this year. Screening and treatment can “improve…
September is Peripheral Artery Disease (PAD) Awareness Month. According to the CardioVascular Coalition, PAD affects approximately 18 million US citizens, and of those, an estimated 160,000 to 180,000 will have a limb amputated this year. Screening and treatment can “improve… 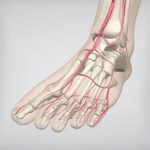 The following story is an example of a coordinated approach to limb salvage instituted at Sanger Heart and Vascular Institute in Charlotte, North Carolina. One of the most devastating consequences of advanced peripheral…
The following story is an example of a coordinated approach to limb salvage instituted at Sanger Heart and Vascular Institute in Charlotte, North Carolina. One of the most devastating consequences of advanced peripheral… 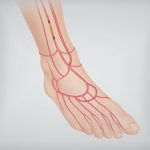 Patients suffering from critical limb ischemia (CLI) are often a difficult population to treat due to the likelihood of significant comorbidities including lesions that may be extremely difficult to cross. Increasingly, physicians are crossing these difficult lesions from a tibiopedal…
Patients suffering from critical limb ischemia (CLI) are often a difficult population to treat due to the likelihood of significant comorbidities including lesions that may be extremely difficult to cross. Increasingly, physicians are crossing these difficult lesions from a tibiopedal… 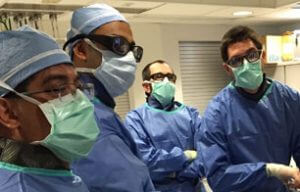 Cook Medical recently participated in a collaborative, first-of-its-kind fellows workshop at the Interventional Radiology−Translational Research and Simulation Lab (IR−TRSL) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The innovative workshop was designed for adult and pediatric IR fellows at the…
Cook Medical recently participated in a collaborative, first-of-its-kind fellows workshop at the Interventional Radiology−Translational Research and Simulation Lab (IR−TRSL) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The innovative workshop was designed for adult and pediatric IR fellows at the… 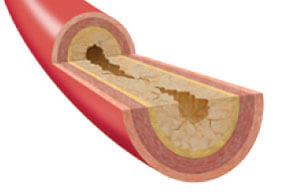 What is PAD? Peripheral arterial disease (PAD) is the result of narrowing of the arteries in the legs, stomach arms, and head. PAD is usually caused by atherosclerosis, a disease that causes arteries to harden due to a buildup of…
What is PAD? Peripheral arterial disease (PAD) is the result of narrowing of the arteries in the legs, stomach arms, and head. PAD is usually caused by atherosclerosis, a disease that causes arteries to harden due to a buildup of…  We recently reached out to Dr. Antonios Gasparis, professor of surgery at Stony Brook University Hospital in New York, to learn more about his hospital’s venous thromboembolism (VTE) team. Upon its formation, the team was tasked with managing patients who…
We recently reached out to Dr. Antonios Gasparis, professor of surgery at Stony Brook University Hospital in New York, to learn more about his hospital’s venous thromboembolism (VTE) team. Upon its formation, the team was tasked with managing patients who…  Challenging times can often be the true test of the strength of a team. In April, our teams faced one of those challenges when we decided to recall all products with Beacon…
Challenging times can often be the true test of the strength of a team. In April, our teams faced one of those challenges when we decided to recall all products with Beacon…  When Joe Roberts began working with venous therapies at Cook Medical, he never imagined that he would develop a deep vein thrombosis (DVT) himself. It's given him a heightened sense of purpose in letting others know about this potentially dangerous condition.
When Joe Roberts began working with venous therapies at Cook Medical, he never imagined that he would develop a deep vein thrombosis (DVT) himself. It's given him a heightened sense of purpose in letting others know about this potentially dangerous condition. 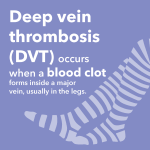 March is DVT Awareness Month. DVT (Deep Vein Thrombosis) can affect people of all ages and can be caused by circumstances that might not be considered as dangerous. Find out some facts that you may not know.
March is DVT Awareness Month. DVT (Deep Vein Thrombosis) can affect people of all ages and can be caused by circumstances that might not be considered as dangerous. Find out some facts that you may not know. 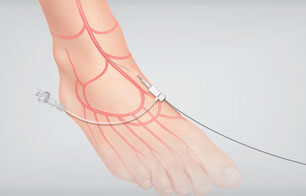 A tibiopedal approach showed 93.5% technical access success and 86.0% technical crossing success in a study sponsored by Cook Medical. Tibiopedal Access for Crossing of Infrainguinal Occlusions (TACO) is the world’s first multicenter, prospective, international study looking at success rates for…
A tibiopedal approach showed 93.5% technical access success and 86.0% technical crossing success in a study sponsored by Cook Medical. Tibiopedal Access for Crossing of Infrainguinal Occlusions (TACO) is the world’s first multicenter, prospective, international study looking at success rates for…  Patients who suffer from peripheral arterial disease (PAD) will often initially visit a podiatrist, who may not be aware of the disease state. To address this educational gap, Peripheral Intervention is sponsoring round tables to help podiatrists learn more about PAD.
Patients who suffer from peripheral arterial disease (PAD) will often initially visit a podiatrist, who may not be aware of the disease state. To address this educational gap, Peripheral Intervention is sponsoring round tables to help podiatrists learn more about PAD.
 Useful for challenging device-implantation cases, the Amplatz Support Wire Guide is now available in a unique tip configuration.
Useful for challenging device-implantation cases, the Amplatz Support Wire Guide is now available in a unique tip configuration.  From a friendship that began more than half a century ago to the modern-day process of bringing new products to market.
From a friendship that began more than half a century ago to the modern-day process of bringing new products to market.  There are now four Micropucture Access Sets with trays. All of these sets include a palladium-tipped, nitinol wire guide and an EchoTip® needle. The sets, in 4 Fr and 5 Fr and standard and stiffened cannulas, also feature Transitionless-Tip™ coaxial…
There are now four Micropucture Access Sets with trays. All of these sets include a palladium-tipped, nitinol wire guide and an EchoTip® needle. The sets, in 4 Fr and 5 Fr and standard and stiffened cannulas, also feature Transitionless-Tip™ coaxial…  Having always been active and a sports enthusiast, Bill Doherty initially thought that the cramping in his left calf was due to muscle strain. He tried physiotherapy, but the frequency and regularity of the cramping became impossible to ignore. He…
Having always been active and a sports enthusiast, Bill Doherty initially thought that the cramping in his left calf was due to muscle strain. He tried physiotherapy, but the frequency and regularity of the cramping became impossible to ignore. He…  Bill Fischer, left, celebrates his 60th wedding anniversary with his wife, Barbara, right, their daughter, Gail McDaniel, and her partner, Keith Hamm. Bill underwent a surgical procedure to treat his advanced peripheral arterial disease. Knowing the symptoms of peripheral arterial…
Bill Fischer, left, celebrates his 60th wedding anniversary with his wife, Barbara, right, their daughter, Gail McDaniel, and her partner, Keith Hamm. Bill underwent a surgical procedure to treat his advanced peripheral arterial disease. Knowing the symptoms of peripheral arterial…  Peripheral arterial disease (PAD) is a serious, underdiagnosed disease that is similar to coronary artery disease. Both develop when cholesterol levels and scar tissue build up, causing the arteries to narrow and restrict blood flow. The difference is that PAD…
Peripheral arterial disease (PAD) is a serious, underdiagnosed disease that is similar to coronary artery disease. Both develop when cholesterol levels and scar tissue build up, causing the arteries to narrow and restrict blood flow. The difference is that PAD…  Nelson L. Bernardo, MD, specializes in interventional cardiology and teaches a course for Vista on retrograde tibiopedal access. Dr. Bernardo is the medical director of the Peripheral Vascular Laboratory at MedStar Heart Institute. He is also the director for…
Nelson L. Bernardo, MD, specializes in interventional cardiology and teaches a course for Vista on retrograde tibiopedal access. Dr. Bernardo is the medical director of the Peripheral Vascular Laboratory at MedStar Heart Institute. He is also the director for… 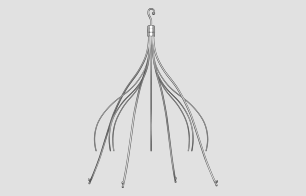 The proven technology of the Cook Celect® Vena Cava Filter is now improved with the addition of four platinum markers. The radiopaque markers on Celect® Platinum can help physicians verify placement accuracy immediately after filter detachment. Celect Platinum features leg struts…
The proven technology of the Cook Celect® Vena Cava Filter is now improved with the addition of four platinum markers. The radiopaque markers on Celect® Platinum can help physicians verify placement accuracy immediately after filter detachment. Celect Platinum features leg struts…  Constantino S. Peña, MD, is an interventional radiologist with Miami Vascular Specialists in Miami, Florida. Dr. Peña received his medical degree from Yale University School of Medicine. He completed an internship in internal medicine at Yale-New Haven Hospital then completed…
Constantino S. Peña, MD, is an interventional radiologist with Miami Vascular Specialists in Miami, Florida. Dr. Peña received his medical degree from Yale University School of Medicine. He completed an internship in internal medicine at Yale-New Haven Hospital then completed… 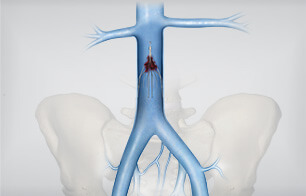 Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava (IVC) filters. The first study, the Cook Inferior Vena Cava Filter (CIVC) study, will add to Cook’s existing clinical…
Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava (IVC) filters. The first study, the Cook Inferior Vena Cava Filter (CIVC) study, will add to Cook’s existing clinical…  We sat down at the recent Venous Symposium meeting in New York City with Dr. Mark Meissner, who shared his thoughts on treating venous disease today and the trends he sees coming in the near future. Dr. Meissner is a…
We sat down at the recent Venous Symposium meeting in New York City with Dr. Mark Meissner, who shared his thoughts on treating venous disease today and the trends he sees coming in the near future. Dr. Meissner is a…  The Advance 35LP Low-Profile PTA Balloon Dilatation Catheter is now available in more options in order to treat lesions of differing lengths. The new balloon lengths are 1.5, 2, 2.5, 17, and 20 cm. The Advance 35LP balloon catheter is…
The Advance 35LP Low-Profile PTA Balloon Dilatation Catheter is now available in more options in order to treat lesions of differing lengths. The new balloon lengths are 1.5, 2, 2.5, 17, and 20 cm. The Advance 35LP balloon catheter is… 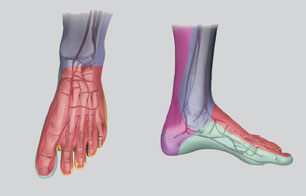 The angiosome concept of perfusion delineates specific vascular territories that are supplied by specific arteries. The concept may help vascular interventionalists treat tissue defects caused by critical limb ischemia (CLI) by enabling the interventionalists to target arteries according to the…
The angiosome concept of perfusion delineates specific vascular territories that are supplied by specific arteries. The concept may help vascular interventionalists treat tissue defects caused by critical limb ischemia (CLI) by enabling the interventionalists to target arteries according to the… 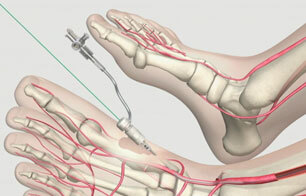 Cook Medical now offers physicians an innovative technology to help treat peripheral arterial disease (PAD) from the knee all the way to the digital arteries. The Advance Micro 14 Ultra Low-Profile PTA Balloon Catheter features a balloon diameter as small…
Cook Medical now offers physicians an innovative technology to help treat peripheral arterial disease (PAD) from the knee all the way to the digital arteries. The Advance Micro 14 Ultra Low-Profile PTA Balloon Catheter features a balloon diameter as small…  Cook Medical's latest addition to its broad offering of wire guides is the Roadrunner® UniGlide.® The hydrophilic UniGlide offers a familiar feel and the performance physicians expect. A durable hydrophilic coating provides trackability and pushability, and a tungsten-impregnated jacket enhances visibility. A…
Cook Medical's latest addition to its broad offering of wire guides is the Roadrunner® UniGlide.® The hydrophilic UniGlide offers a familiar feel and the performance physicians expect. A durable hydrophilic coating provides trackability and pushability, and a tungsten-impregnated jacket enhances visibility. A…  The ultra low-profile of Cook Medical's latest balloon allows physicians to attempt interventions that they never thought were possible. The Advance Micro™ 14 Ultra Low-Profile PTA Balloon Catheter is designed specifically to treat vessels from the knee all the way…
The ultra low-profile of Cook Medical's latest balloon allows physicians to attempt interventions that they never thought were possible. The Advance Micro™ 14 Ultra Low-Profile PTA Balloon Catheter is designed specifically to treat vessels from the knee all the way… 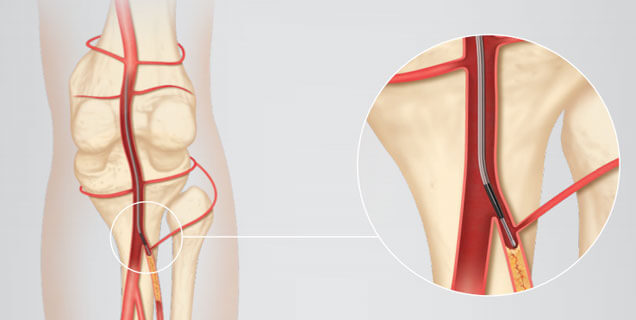 The CXI® Support Catheter is engineered for the periphery and can help you reach and cross lesions.CXI is now available in an .014 inch platform that features platinum-iridium marker bands for exceptional visibility. CXI’s stainless steel braid provides a 1:1 torque…
The CXI® Support Catheter is engineered for the periphery and can help you reach and cross lesions.CXI is now available in an .014 inch platform that features platinum-iridium marker bands for exceptional visibility. CXI’s stainless steel braid provides a 1:1 torque…  The Advance® 18LP Low-Profile PTA Balloon Dilatation Catheter is now available in nearly 200 size, length, and shaft configuration options. Additional balloon diameters are 2, 9, and 10 mm, while additional lengths are 12, 15, 17, and 20 cm. A catheter…
The Advance® 18LP Low-Profile PTA Balloon Dilatation Catheter is now available in nearly 200 size, length, and shaft configuration options. Additional balloon diameters are 2, 9, and 10 mm, while additional lengths are 12, 15, 17, and 20 cm. A catheter…  Dr. Larisee Lee, a physician from Southern California, talks about her experience attending a course through Cook Medical’s Vista℠Collaboration and Learning Program. We're confident you'll find an equally productive experience, whether you are just getting started or simply polishing your…
Dr. Larisee Lee, a physician from Southern California, talks about her experience attending a course through Cook Medical’s Vista℠Collaboration and Learning Program. We're confident you'll find an equally productive experience, whether you are just getting started or simply polishing your…  [brightcove 2483297889001] Retrograde tibiopedal access is a new approach to treating the growing problem of peripheral arterial disease (PAD) and critical limb ischemia. Many physicians are discovering this technique that offers another method of crossing lesions when an attempt to cross…
[brightcove 2483297889001] Retrograde tibiopedal access is a new approach to treating the growing problem of peripheral arterial disease (PAD) and critical limb ischemia. Many physicians are discovering this technique that offers another method of crossing lesions when an attempt to cross…  The Celect Platinum is designed to prevent recurrent pulmonary embolism (PE) via placement in the inferior vena cava. The Celect Platinum can be used as either a permanent or a retrievable device. Its secondary strut design is intended to help…
The Celect Platinum is designed to prevent recurrent pulmonary embolism (PE) via placement in the inferior vena cava. The Celect Platinum can be used as either a permanent or a retrievable device. Its secondary strut design is intended to help…